How To Use Precipitation, Not Protein A Chromatography, For mAb Capture
By Andrew Zydney, Ph.D., Department of Chemical Engineering, The Pennsylvania State University

Precipitation was one of the earliest techniques used for protein separations, dating back to the pioneering work by Antoine Fourcroy that led to the identification of proteins like albumin, fibrin, and gelatin.1 Fractional precipitation is also the basis for the large-scale purification of plasma proteins using the Cohn fractionation process that was originally developed in the 1940s and is still used today.2 However, the early recombinant proteins were produced at concentrations <100 mg/L, making precipitation difficult if not possible. The platform process that evolved for monoclonal antibody (mAb) manufacturing is instead based on the use of Protein A affinity chromatography for the initial capture step, providing both purification and significant mAb concentration in a single step.3 Although Protein A chromatography is highly efficient and robust, it has also been identified as a key bottleneck in mAb manufacturing due to the high cost of the resin and the limited throughput of bind-and-elute chromatography.
Recent advances in upstream processing, including improvements in cell culture media and bioreactor design, have resulted in titers approaching, and in some cases exceeding, 10 g/L, conditions that can easily accommodate precipitation as an alternative for initial mAb capture. Several groups have thus begun exploring the potential of using precipitation in downstream processing of biopharmaceuticals.4 We have recently demonstrated that it is possible to precipitate mAbs directly from clarified cell culture fluid (CCF) using a combination of ZnCl2, a reversible cross-linking agent, and polyethyleneglycol (PEG), a volume exclusion agent that acts synergistically with the Zn.5 Highly selective mAb precipitation with easy redissolution can be achieved by pretreating the cell culture fluid to remove DNA, which can also be performed by precipitation but using CaCl2 as the precipitant. This approach has been successfully employed for more than a dozen commercial mAbs from a variety of industrial partners, with only slight adjustments in the ZnCl2 and PEG concentrations needed based on the specific properties of the mAb. Optimal precipitation conditions are easily identified using high-throughput screening experiments that require minimal amounts of protein.6
Continuous Precipitation-Filtration Process
Although precipitation is often thought of as a batch process, mAb precipitation can be readily implemented in a continuous format using simple tubular precipitation reactors employing static mixers inside of appropriately sized plastic tubing. The static mixers provide relatively narrow residence time distributions, leading to a very uniform microenvironment that is needed to achieve the desired product quality and process control. The ZnCl2 and PEG can be added sequentially using tubular precipitation reactors in series to optimize the nucleation and growth phases to obtain the desired precipitate morphology.
High degrees of host cell protein (HCP) removal can then be achieved by dewatering and washing the precipitate using hollow fiber tangential flow microfiltration modules operated continuously in single pass mode at high conversion (i.e., high ratio of permeate to feed flow rate, typically greater than 60%). The membrane modules are staged in a countercurrent configuration to maximize the extent of HCP removal while minimizing the amount of buffer required for effective washing, enabling a significant reduction in process mass intensity (PMI) relative to the platform Protein A process. The concentrated and washed precipitate can then be redissolved by lowering the solution pH to less than 4.5, enabling direct integration with a low pH virus inactivation step followed by polishing operations to reduce the final HCP and DNA concentrations to below the targeted values. The polishing steps can potentially be performed using flow-through chromatography, e.g., in low-cost membrane adsorbers, resulting in a fully continuous downstream process that can be directly integrated with high productivity perfusion bioreactors that are currently being deployed for continuous upstream processing (Figure 1).
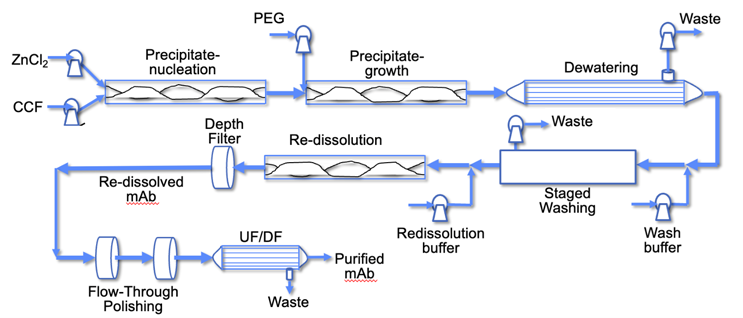
Figure 1 – Schematic of the key steps in a fully continuous downstream mAb purification process based on precipitation-filtration with countercurrent staged washing and flow-through polishing operations.
Challenges And Opportunities
Although mAbs are readily precipitated using Zn+2 as a reversible crosslinking agent, there are a number of components in the cell culture media that can inhibit this process, including the formation of insoluble zinc phosphate. We have found that it is often necessary to precondition the CCF by continuous diafiltration or countercurrent dialysis5 to remove these small interfering components to achieve high yields of the mAb precipitate at manageable (<10 mM) Zn concentrations. Although this preconditioning requires an additional processing step, it does provide an opportunity to standardize the precipitation process for a wider range of cell culture conditions, which could facilitate the development of a new platform process for mAb purification.
Successful application of this continuous precipitation-filtration technology also requires that the membrane modules be operated under conditions that minimize membrane fouling. This can be achieved by operating below the critical filtrate flux, which is defined as the maximum value of the filtrate flux at which membrane fouling is negligible. However, it can often be difficult to achieve high conversions while still operating below the critical flux. It is always possible to increase the degree of HCP removal by using more stages in the countercurrent washing step, but this adds to both system complexity and overall cost of manufacturing.
Recent work in our laboratory, supported by a contract from the U.S. FDA, has shown for the first time that it is possible to increase the critical flux by adjusting the pH and/or by adding CaCl2 to control the morphology of the protein precipitate.7 More compact/dense precipitates lead to higher critical flux, allowing one to operate the hollow fiber modules continuously and at high conversion for multiple days with minimal fouling and thus without any membrane cleaning or regeneration. The higher critical flux also reduces the required membrane area for each module, providing further improvements in the overall process economics. Future efforts will be needed to more fully understand the relationship between the precipitate morphology and the filtration behavior, but this approach could potentially lead to the development of novel strategies to enhance the performance of the tangential flow filtration modules used for dewatering and washing.
There is growing concern that the current Protein A processing platform will be unable to meet the growing global demand for mAb products, both in low- and middle-income countries and for new disease targets with very large patient populations like the treatment of Alzheimer’s and high cholesterol. Continuous precipitation coupled with tangential flow filtration could provide a highly attractive alternative with much lower cost of goods and easy scalability. The ability to achieve highly selective mAb precipitation with only small adjustments in conditions should support the rapid development timelines needed for mAb products, and the lower PMI should lead to more sustainable manufacturing processes. The resulting precipitation-based process could provide a framework for the development of a truly continuous biomanufacturing platform that can support the production of mAbs for the coming decades.
References
- Tanford, C., Reynolds, J., Nature’s Robots – A History of Proteins, Oxford University Press, United Kingdom (2001).
- Cohn, E.J., Strong, L.E., Hughes, W.L., Mulford, D.J., Ashworth, J.MN., Melin, M., Taylor, H.L., Preparation and properties of serum and plasma proteins; a system for the separation into fractions of the protein and lipoprotein components of biological tissues and fluids. J Am Chem Soc. 68: 459-75 (1946).
- Shukla A, Hubbard B, Tressel T, Guhan S, Low D., Downstream processing of monoclonal antibodies–application of platform approaches. J Chromatogr B. 848: 28–39 (2007).
- dos Santos, R., Carvalho, A.L., Roque, A.C.A., Renaissance of protein crystallization and precipitation in biopharmaceuticals purification, Biotechnol Adv 35: 41-50 (2017).
- Minervini, M., Mergy, M., Zhu, Y., Guttierez Diaz, M.A., Pointer, C., Shinkazh, O., Oppenheim, S.F., Cramer, S.M., Przybycien, T.M., Zydney, A.L., Continuous precipitation-filtration process for initial capture of a monoclonal antibody product using a four-stage countercurrent hollow fiber membrane step, Biotech Bioeng, (2013). https://doi.org/10.1002/bit.28525
- Gu, Q., Li, Z., Coffman, J.L., Przybycien, T.M., Zydney, A.L. High throughput solubility and redissolution screening for antibody purification via combined PEG and zinc chloride precipitation,” Biotech Prog. 36(6): e3041 (2020).
- Minervini, M., Behboudi, A., Marzella, J.R., Zydney, A.L., Optimizing tangential flow filtration performance by controlling the morphology of precipitated proteins, Sep Purif Tech (under review).
About The Author:
 Andrew L. Zydney, Ph.D., is the Bayard D. Kunkle Chair and Professor of Chemical Engineering at The Pennsylvania State University. Zydney also serves as director of the Penn State site in the Membrane Applications, Science, and Technology (MAST) Center. Professor Zydney's research is focused on the application of membranes in bioprocessing, including the purification of monoclonal antibodies, vaccines, and gene therapy agents. He is a recent recipient of the Alan S. Michaels Award for Innovation in Membrane Society and Technology (from the North American Membrane Society) and the ACS Separations Science and Technology Award (from the American Chemical Society). He served as editor-in-chief of the Journal of Membrane Science for more than 10 years and is past president of the North American Membrane Society (NAMS). Zydney serves as Chair of the Technical Advisory Board for Chromatan Corp., a startup company pursuing the commercialization of continuous countercurrent tangential chromatography for biomanufacturing.
Andrew L. Zydney, Ph.D., is the Bayard D. Kunkle Chair and Professor of Chemical Engineering at The Pennsylvania State University. Zydney also serves as director of the Penn State site in the Membrane Applications, Science, and Technology (MAST) Center. Professor Zydney's research is focused on the application of membranes in bioprocessing, including the purification of monoclonal antibodies, vaccines, and gene therapy agents. He is a recent recipient of the Alan S. Michaels Award for Innovation in Membrane Society and Technology (from the North American Membrane Society) and the ACS Separations Science and Technology Award (from the American Chemical Society). He served as editor-in-chief of the Journal of Membrane Science for more than 10 years and is past president of the North American Membrane Society (NAMS). Zydney serves as Chair of the Technical Advisory Board for Chromatan Corp., a startup company pursuing the commercialization of continuous countercurrent tangential chromatography for biomanufacturing.
