How To Implement Size Exclusion Chromatography And Mitigate HCP Risk
By Younghoon Oh, Ph.D., Janssen
Host cell proteins (HCPs) are process-related impurities that form during the production of cell culture-based biologics such as therapeutic proteins, certain types of vaccines, and cell and gene therapies. HCP concentration is a critical quality attribute (CQA) for most biologics, including mAbs and vaccines. Many studies have been reported in the literature to characterize the persistence of HCPs in purification steps and to subsequently improve HCP mitigation strategies. While HCP enzyme-linked immunosorbent assay (ELISA) serves as the industry standard in determining the level of HCPs for lot release testing, advancement and implementation of liquid chromatography-tandem mass spectrometry (LC-MS/MS) enabled identification and quantitation of individual HCPs present in the process intermediates and drug substance above the assay threshold as an orthogonal characterization method.
Enhanced Profiling Of Process-related Impurities By SEC
A group of scientists from the Bioprocessing Technology Institute in Singapore published their findings between 2013 and 2015 using size-exclusion chromatography (SEC) and impurity studies.1-3 They found that DNA and HCPs are biasedly distributed in the SEC fractions of clarified harvest, with high molecular weight aggregate fractions (HMWAFs) being identified as a reservoir of, specifically, chromatin-derived complexes made of histones, non-histone HCPs, DNA and IgGs or IgMs. Such chromatin aggregates were found to be challenging to remove during protein A chromatography due to nonspecific interactions between the aggregates and protein A ligands.
Different scientific groups have recently revived the method of size-based impurity segregation and characterization; this time, LC-MS/MS is used for HCP identification and quantitation in the SEC fractions of the intermediates of the mAb process.4-6 The importance of this strategy in gaining access to a more thorough HCP profile and, thus, understanding HCP persistence in the purification processes, will be emphasized in the following subsections.
Improved HCP Detection Capability
Fractionation of the process intermediates by SEC greatly helps improve HCP detection capability of LC-MS/MS. In other words, many more HCPs may be detected when fractionated samples are analyzed relative to unfractionated samples. The number of HCPs detected was 1.3 to 2.9 times greater when SEC was conducted prior to LC-MS/MS than when no fractionation was performed for eight different clarified harvest samples.5,7 Such discrepancy in HCP detection capability between the two approaches remains consistent or even becomes greater with purified samples. For a protein A eluate,7 two first polishing pools,5 and a drug substance6 analyzed, the number of HCPs detected was 2.6 to 16 times greater by the SEC-based method than by the control method.
All these results show that implementation of SEC can greatly improve the detection capability of HCPs by LC-MS/MS, especially with HMWAF identified as having more HCPs identified than any other fractions or even unfractionated raw intermediates, suggesting the limit of the current standard method in the detection capability.
Uneven Distribution Of HCPs In The SEC Fractions
Categorization of HCPs and other impurities based on their occurrence in the SEC fractions allowed for the identification of specific characteristics of mAb process intermediates, such as uneven distribution of HCPs and DNA in the samples, which is partly driven by the innate characteristics of such impurities for their functionality required for proper cellular processes.1-3,5 In that regard, there is a likely relationship between the presence of certain types of HCPs in the SEC fractions and their functional roles (for example, proteins involved in protein synthesis and degradation are abundantly present in HMWAF and/or main-monomer fractions, indicating potential association of such proteins with the product).5
This observation seems to suggest that we shift our view from considering HCPs to be inert objects that are defined just by protein sequence and a few physicochemical qualities to perceiving them as functional and dynamic entities that are essential to various cellular processes, including synthesis and controlling level of the product. Furthermore, the larger HCPs found in the LMWF raise the possibility that there may be HCPs in degraded forms.5
All these illustrate the intricacy of mAb process intermediates and the fact that developing efficient HCP removal strategies may require knowledge of the precise types of HCPs that may not be readily apparent with the conventional method of MS-based HCP detection in raw process streams.
Identification Of mAb-associated HCPs
The knowledge of the occurrence of HCPs in the SEC fractions was used to identify the binding properties of HCPs to a mAb. Zhao et al.6 implemented two SEC operating conditions to create the HMWAF, main-monomer fraction, and two low molecular weight fractions (LMWFs):
Native SEC— SEC carried out under conditions that could preserve the original HCP-mAb interactions
Mild-denaturing SEC — SEC carried out under conditions that could disrupt HCP-mAb interactions
HCPs found in HMWAF were assumed to be those that were bound to the mAb, whereas HCPs found in LMWFs were not.
According to this supposition, HCPs seen in HMWAF but not in LMWFs under the two SEC circumstances were those that were tightly bound to the mAb. Conversely, HCPs found solely in LMWFs of the mild-denaturing SEC but only in HMWAF of the native SEC were hypothesized to be those weakly bound to the mAb.
Another method of using information about the occurrence of HCPs in SEC fractions to identify mAb-associated HCPs relies on a different theory. HCPs that elute with monomer during SEC are those that are most likely bound to the mAb.5 The monomer fraction of the two earlier intermediates (i.e., protein A eluate and clarified harvest) of two mAbs consistently contained nine HCPs that also eluted with monomer during SEC of the first polishing pool of the two mAbs, eight of which were previously reported to be associated with multiple mAbs. The outcome of a further experiment, in which protein A chromatography was carried out with each of the four SEC fractions from a clarified harvest of a mAb (bigger HMWAF, smaller HMWAF, main-monomer, and LMWF), further corroborated this observation.8
While 44% of the HCPs in the harvest's main-monomer fraction persisted after protein A chromatography, just 1.9% to 5.9% of the HCPs in the harvest's other SEC fractions did. Given that mAb-HCP association was proposed to be the controlling mechanism of HCP persistence during this process, these findings support the theory that HCPs that co-elute with monomer during SEC are more likely those bound to the product.
Both studies demonstrate how SEC can be utilized in conjunction with LC-MS/MS analysis to comprehend and analyze HCP persistence, particularly for the detection of mAb-associated HCPs, in mAb processing steps, even though evaluation of the two techniques may necessitate future research.
Tracking The Origins Of HCPs
Along with identifying mAb-associated HCPs, this technique can be used to trace the origins of HCPs between two adjacent purification process steps.
Figure 1 shows potential occurrences of a persistent HCP in three SEC fractions for two successive intermediates of purification steps. The number of possibilities where the HCP can be found among the three SEC (i.e., HMWAF, main-monomer, LMWF) is seven.
The HCP can then be transferred from the intermediate of purification step 1 to the intermediate of purification step 2 in "7 x 7 = 49" circumstances. This case appears to be very complicated, but because many HCPs appear to be particular to one fraction (for instance, some HCPs can only be present in HMWAF or LMWF of both intermediates),5 this method can help explain these HCPs' properties and their persistent mechanisms to aid in the development of removal strategies that are tailored to them.
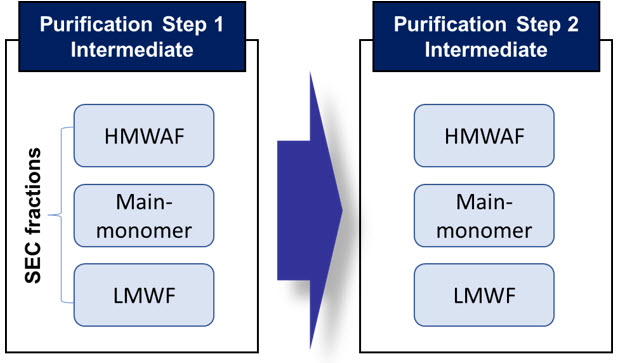
Figure 1. Possible occurrence of HCPs in the SEC fractions of two subsequent process intermediates. An HCP can be found in only one of the three fractions (three possibilities), two of the fractions (three possibilities) or all three fractions (one possibility) in each step.
Beyond mAb Manufacturing
Most of these key findings and lessons learned from this methodology for characterizing the HCP profile in mAb process intermediates might also apply to other cell culture-based biological modalities.
For example, in terms of the composition of product-impurity aggregates and concerns about the difficulties in controlling such impurities, a recent study on column reusability for adeno-associated vaccine (AAV) production for gene therapy applications reveals similarities between AAV process intermediates and mAb intermediates.9 In addition, the adeno viral vector-based ChAdOx1 nCov-19, a vaccine approved for SARS-CoV-2, was found to contain higher levels of HCPs in the four tested lots.10 The 20 abundant HCPs detected in the vaccine lots were searched against the mAb-based SEC-HCP proteome database by Oh et al.5 to conjecture where these HCPs might be found in the clarified harvest and the affinity chromatography eluate (the search was restricted to the 11 common HCPs found both in the vaccine lots and the mAb database). With the exception of one HCP, the HMWAF was shown to be the predominant residence of these HCPs in the mAb clarified harvest. Also, among seven different mAb protein A eluate samples investigated, these 11 HCPs showed up frequently in HMWAF. These findings would suggest that HMWAF is a potential primary source of such HCPs in DSP intermediates. However, such an analysis must be carried out with caution because there is a risk involved in extrapolating data from a mAb-based database to describe the traits of a different process and sample.
Conclusion
The use of SEC in conjunction with MS analysis in recent studies has increased our knowledge of the HCP profile and its potential and implications for developing successful HCP-mitigation strategies. Further application of this methodology to other cell culture-based biologics is required for a thorough evaluation of its advantages, and it is anticipated that doing so will lead to new discoveries and advancement of our knowledge on HCP persistence for the broader biopharmaceutical community.
The author wants to thank Michael Capaldi, Dr. Nicholas Levy, Dr. Kristopher Barnthouse, Dr. Carmelata Chitikila, and Dr. Jingjie Mo for their review and suggestions on this article.
References
- Gagnon, P., Nian, R., Lee, J., et al. (2014). Nonspecific interactions of chromatin with immunoglobulin G and protein A, and their impact on purification performance. Journal of Chromatography A, 1340: 68–78.
- Gagnon, P., Nian, R., Yang, Y., et al. (2015). Nonimmunospecific association of immunoglobulin G with chromatin during elution from protein A inflates host contamination, aggregate content, and antibody loss. Journal of Chromatography A, 1408: 151–160.
- Gan, H.T., Lee, J., Latiff, S.M.A., et al. (2013). Characterization and removal of aggregates formed by nonspecific interaction of IgM monoclonal antibodies with chromatin catabolites during cell culture production. Journal of Chromatography A, 1291: 33–40.
- Hu, M., Molden, R., Hu, Y., et al. (2022) Host cell protein identification in monoclonal antibody high molecular weight species. Journal of Chromatography B, 1210: 123448.
- Oh, Y.H, Becker, M.L., Mendola, K.M., et al. (2023) Characterization and implications of host-cell protein aggregates in biopharmaceutical processing. Biotechnology and Bioengineering, 120(4): 1068-1080.
- Zhao, B., Abdubek, P., Zhang, S., et al. (2022) Analysis of host cell proteins in monoclonal antibody therapeutics through size exclusion chromatography. Pharmaceutical Research, 39: 3029-3037.
- Herman, C., Min, L., Choe, L.H., et al. (2023) Analytical characterization of host-cell-protein-rich aggregates in monoclonal antibody solutions, Biotechnology Progress, 39(4): e3343.
- Oh, Y.H. (2022) Mechanisms of host-cell protein persistence in monoclonal antibody processing. Ph.D. Thesis (Chapter 4.3.4). University of Delaware.
- Soni, H., Lako, I., Placidi, M., et al. (2023) Implications of AAV affinity column reuse and vector stability on product quality attributes. Biotechnology and Bioengineering, doi: 10.1002/bit.28500
About the Author:
 Younghoon Oh, Ph.D., is a scientist of Janssen R&D of Johnson & Johnson. Prior to his involvement in the current subject, Younghoon had worked in the fields of microbial metabolic engineering and fermentation, contributing to the publishing of over 30 scientific papers and five inventions. He explored the mechanisms of HCP persistence using proteomics analysis while receiving his Ph.D. in chemical and biomolecular engineering from the University of Delaware and working under Abraham M. Lenhoff, Ph.D. Since joining Janssen R&D, he has continually worked on HCP persistence and downstream process development for mAb-based products.
Younghoon Oh, Ph.D., is a scientist of Janssen R&D of Johnson & Johnson. Prior to his involvement in the current subject, Younghoon had worked in the fields of microbial metabolic engineering and fermentation, contributing to the publishing of over 30 scientific papers and five inventions. He explored the mechanisms of HCP persistence using proteomics analysis while receiving his Ph.D. in chemical and biomolecular engineering from the University of Delaware and working under Abraham M. Lenhoff, Ph.D. Since joining Janssen R&D, he has continually worked on HCP persistence and downstream process development for mAb-based products.
