How Takeda Streamlines Assay Transfer And Troubleshooting With The Artel MVS®
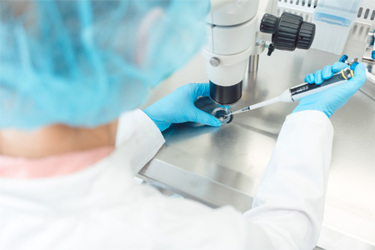
Takeda’s quality control (QC) facility in Lexington, MA, constantly runs dozens of different assays of varying complexity as part of the product release process. When an assay fails, the QC team launches an investigation to determine the cause and prevent a reoccurrence of the same error.
NorDx has consistently prioritized quality, accuracy, and efficiency. As the company expanded from a single hospital lab to twelve sites, it had to overcome the challenge of ensuring consistency and comparability of test data and results across all locations. This challenge has only been further intensified by the nationwide shortage of qualified and certified medical technologists.
The QC team turned to the Artel MVS® for help. They utilized the instrument to promptly identify whether assay failure resulted from variability in human pipetting or the automated liquid handlers being out of spec. Explore the overall impact of this solution on Takeda’s QC facility as well as the results from this study.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Bioprocess Online? Subscribe today.
