Global Market Outlook For Cancer Biological Therapies
By Rupali Swain and Subodh Kharad, Global Market Insights
According to the World Health Organization, in 2020 cancer was responsible for nearly 10 million deaths, with breast, colon, lung, rectum, and prostate cancers among the most prevalent types. Based on this trend, the National Cancer Institute estimates that the number of new cancer cases per year is expected to rise to 29.5 million by 2040, while the number of cancer-related deaths will be close to 16.4 million.
Traditional cancer treatments like chemotherapy and radiation have long been used to treat these conditions, but they come with severe side effects that last for months or even years after the treatment is completed. Therefore, as a safer alternative, patients are preferring cancer biological therapy due to its fewer toxic side effects compared to chemotherapy and its ability to prevent or slow down tumor growth and block the spread of cancer while doing less harm to healthy cells.
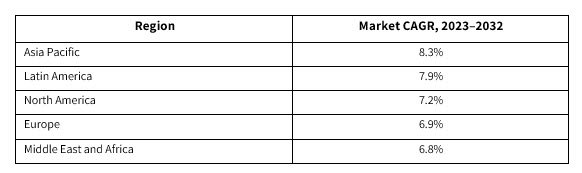
Our new research estimates that the cancer biological therapy market will be valued at over $277 billion by 2032. Geographically, forecasted market growth indicates:
By route of administration, the cancer biological therapy market is broadly segmented into injectable and oral. The injectable and oral segments are expected to reach $95.9 billion and $131.5 billion, respectively, by the end of 2032.
The notable players in the market include AbbVie, Merck, AstraZeneca, GlaxoSmithKline, Eli Lilly and Company, Otsuka, Pfizer, Hoffmann-La Roche, Novartis, and Takeda Pharmaceuticals.
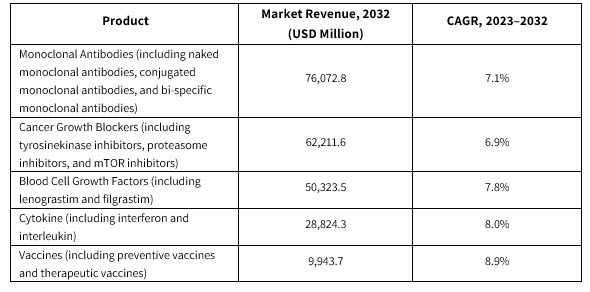
Forecasted growth in the market’s segments include:
Let’s delve into three of the most notable segments: monoclonal antibodies, cancer growth blockers, and cancer vaccines.
1. Monoclonal Antibodies
Monoclonal antibodies are efficient and nontoxic in the diagnosis and treatment of different types of cancer when compared to chemotherapy and other cancer treatment methods. Moreover, getting approvals for many new cancer monoclonal antibodies from the respective authorities is more efficient and easier compared to other therapies. For instance, in January 2021, the antibody-drug conjugate trastuzumab deruxtecan was granted conditional approval in the European Union for use as a single agent in the treatment of adult patients with unresectable or metastatic HER2-positive breast cancer. A rise in likely regulatory approval of other cancer monoclonal antibodies will fuel growth in the market.
2. Cancer Growth Blockers
Cancer growth blockers, also often referred to as cancer growth inhibitors, are targeted drugs that work by blocking the growth factors that trigger cancer cells to divide and grow. The benefits these drugs hold include lower side effects than traditional treatments, targeted therapy, and the ability to be used in combination with chemotherapy and radiotherapy.
Tyrosine kinase inhibitors (TKIs), among the most effective cancer growth blockers, are used to treat many types of cancer, including gastrointestinal stromal tumors, kidney cancer, HER2-positive breast cancer, and brain-metastatic breast cancer. They work by blocking chemical messengers called tyrosine kinases that help to send growth signals in cancer cells. The U.S. is emerging as a lucrative market for TKI drugs. For instance, estimates suggest that the commercial market for TKI in the U.S. has reached $3.5 billion and has grown by nearly 5% since 2020.
The cancer growth blockers segment is expected to show lucrative growth of 6.9% to reach $62.2 billion by 2032.
Some of the latest developments in cancer growth blockers include:
- In February 2023, Xspray Pharma AB signed an agreement with EVERSANA to support the launch and commercialization of its first innovative cancer therapy Dasynoc for the treatment of chronic myeloid leukemia (CML) and acute lymphatic leukemia (ALL) in the U.S.
- In January 2023, Alpha Biopharma announced that China’s Center for Drug Evaluation of the National Medical Products Administration has approved a new drug application (NDA) for zorifertinib, a next-generation EGFR tyrosine kinase inhibitor (TKI), for patients with advanced EGFR-mutated NSCLC (non-small cell lung cancer) with central nervous system (CNS) metastases.
3. Cancer Vaccines
Cancer vaccines are shown to be effective in preventing certain types of cancer, including cervical cancer, which is primarily caused due to human papillomavirus (HPV). These vaccines boost the immune system's ability to find and destroy antigens.
Elevated cases of HPV-associated cancers could drive the uptake of these cancer vaccines in the years to come. A report from the Centers for Disease Control and Prevention (CDC) claims that around 47,199 new HPV-associated cancers occur in the U.S. each year, of which 26,177 are among women and 21,022 are among men. Health agencies like the FDA have recently approved HPV vaccines for other cancers such as vaginal, vulvar, and anal cancer.
Work is currently underway to obtain approval for vaccines for oral cancer and several clinical trials and research studies are being conducted to further develop other cancer vaccines.
The vaccines segment is expected to reach $9.9 billion by 2032. The key players manufacturing cancer vaccines include GlaxoSmithKline and Merck.
Some of the latest developments in cancer vaccines include:
- In February 2023, German biotechnology company BioNTech revealed plans to initiate clinical trials in Britain for its cancer vaccines that target breast, lung, and pancreatic cancer. The company aims to provide personalized cancer therapies for around 10,000 patients by the end of 2030 in either a clinical trial setting or as an approved treatment.
- In February 2023, Indian pharmaceutical company MSD Pharmaceuticals hinted at its participation in a global tender to procure HPV vaccines against cervical cancer for the immunization of girls aged 9 to 14 years. The tender is expected to be proposed by India’s Union Health Ministry for procuring 160 million doses of HPV vaccines by 2026.
The Future Of Cancer Biological Therapies
The future of cancer biological therapies looks promising given a number of new advancements, such as the development of CAR-T therapy, gene therapy, and monoclonal antibodies, and the limited effectiveness of chemotherapy. Researchers are adding emphasis on improving the effectiveness of biological therapy for cancer and lowering its potential side effects.
CAR T cell therapy: Cancer cells are known to hide from the normal immune system, but through CAR T cell therapy, scientists and professionals can make T cells better equipped to find and kill some cancer cells. Therefore, an increase in cancer cases is anticipated to drive the demand for CAR T cell therapy. Additionally, increasing research and development are contributing to market growth. For instance, in December 2021, Novartis reported the introduction of T-Charge, a next-generation CAR-T platform that aims to serve as the foundation for various new investigational CAR T cell therapies in the Novartis pipeline.
Gene therapy: These include anti-angiogenic gene therapy, pro-drug activating suicide gene therapy, gene therapy-based immune modulation, oncolytic virotherapy, correction/compensation of gene defects, antisense, genetic manipulation of apoptotic and tumor invasion pathways, and RNAi strategies. Oncolytic viruses can combat cancer cells without disturbing the healthy cells in the vicinity by stimulating natural killer cells. Moreover, there are lucrative research grants for research on oncolytic virotherapy. For instance, in July 2022, the researchers at the Center for Nuclear Receptors and Cell Signaling at the University of Houston received a $1.8 million grant from the National Institutes of Health to work on oncolytic virotherapy.
