Gene Beat VII
Gene for Fastest Known Biological Motor Cloned
First Human Disorder Linked To A Chaperonin
Unusual Tumor Suppressor Gene Found
High Anxiety
Gene for Fastest Known Biological Motor Cloned (Back to Top)
In an unusual collaboration between hearing scientists and molecular biologists, researchers at Northwestern University (Evanston, IL) have cloned a gene that is critical to the functioning of the outer hair cell, a sensory receptor cell unique to the inner ear of mammals. As reported in the May 11 issue of the journal Nature, Peter Dallos, professor of neuroscience, and Laird D. Madison, assistant professor at the Center for Endocrinology, Metabolism and Medicine, have successfully cloned the gene prestin, which codes for a protein (prestin). Prestin, named after the musical term presto, is the fastest molecular motor known, causing the expansion and contraction of the outer hair cells at the frequency of the incoming sound.
Prestin is unique among molecular motors in that, unlike the other known motors—kinesin, dynein, and myosin—it does not require ATP, but responds directly to voltage. Hence, these tiny biocompatible motors could be a boon to the developing nanotechnology field.
"Here we have a molecule that directly converts electricity into mechanical force," said Dallos. "This novel motor potentially could be used to build machines on the molecular scale." The other biological motors are poor candidates because they are slow and do require additional energy to work
Outer hair cells act as local mechanical amplifiers of the incoming sound vibrations, giving the mammalian ear its extraordinary sensitivity and frequency-resolving capacity. During amplification, the cylinder-shaped outer hair cells elongate and contract at the same very rapid rate as the frequency of the incoming sound. They boost the signals received by the inner hair cells, the sensory cells located in the cochlea that are responsible for communicating with the central nervous system. The inner hair cells send the auditory information to the brain, which interprets it.
Believing that a molecular motor was responsible for the changes in the cell's length, the researchers set out to find the motor gene. To do this, they compared the genetic read-out of inner versus outer hair cells, which are quite similar except that inner hair cells are non-motile. Using subtractive hybridization, they identified a small cohort of genes expressed only in outer hair cells, from which they chose a likely candidate.
They next transfected their candidate gene into cultured human kidney cells, which do not exhibit microscopic movement. When the prestin gene was expressed in the transfected cells, the cells responded to applied voltages, proving that they had found the molecular motor.
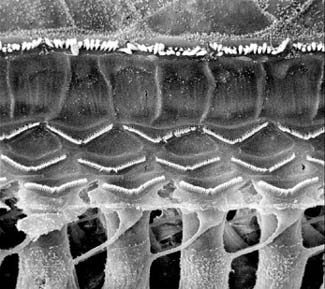
Scanning electron micrograph of the structural organization of the hair cells of the inner ear. The white tufts in this picture are the sensory hairs (stereocilia) on the top of each cell—hence the name "hair cell." The upper row represents the hairs of the "inner hair cells" and the three rows of w-shaped hairs indicate the position of the "outer hair cells."
Zheng, J., et al., "Prestin is the motor protein of cochlear outer hair cells," Nature 405: 149-155, 2000.
First Human Disorder Linked to A Chaperonin (Back to Top)
Scientists at the National Human Genome Research Institute (NHGRI) and the University of Michigan Medical Center (Ann Arbor) have found that a mutation in a chaperonin causes a rare developmental syndrome called McKusick-Kaufman syndrome (MKS), found predominantly among the Old Order Amish population. As reported in the May issue of Nature Genetics, Leslie Biesecker and colleagues used a technique called sample sequencing to find the gene among 450 kilobases of sequence on chromosome 20, which they determined contained the gene based on family studies.
Females with MKS are affected by hydrometrocolpos (accumulation of fluids in the uterus and vagina), which in neonates can cause fatal lung compression. Both males and females have a form of polydactly (the presence of extra fingers or toes) and congenital heart disease.
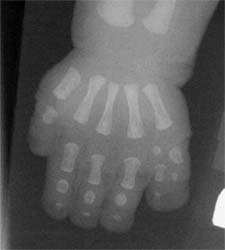
Polydactly—the presence of extra fingers or toes—is a trait found in both males and females with MKS.
Focusing on one gene that was expressed to high levels in MK patients, the researchers found both missense and nonsense mutations in the gene, which bears sequence similarity to a member of the chaperonin family found in the thermophile Thermus acidophilus. Although the function of the protein made by the gene is unclear, the researchers speculate that it may be involved in the processing of proteins involved with development of limbs, the heart, and the reproductive system.
"The MKKS gene mutations suggest that protein integrity may be critical to developmental processes," said Biesecker. "By studying these rare or so-called orphan diseases, we learn more about both normal and abnormal human development," he added. "We are grateful to the Amish families who assisted us to find this gene. It is hoped that we can develop a screening process for carrier couples so that their pregnancies can be monitored for hydrometrocolpos by ultrasound. Affected girls could be delivered in a setting that would allow rapid surgical correction that could be life saving."
Stone, DL, et al., "Mutation of a gene encoding a putative chaperonin causes McKusick-Kaufman syndrome." Nature Genetics 25 (1): 79-82, 2000.
Unusual Tumor Suppressor Gene Found (Back to Top)
Michael Stratton of the Institute of Cancer Research (Surrey, UK) has identified the gene for familial cylindromatosis, an autosomal dominant genetic disorder. This disease, better known as "turban tumor," causes small, cylindrical tumors on the surface layers of the skin on the neck and scalp. While in the main benign, these tumors occasionally become infected and ulcerated, and can cause severe disfigurement and discomfort.
The susceptibility gene (cyld), previously localized on chromosome 16, has now been analyzed for both germline mutations and for somatic mutations in familial and sporadic cylindromas. While the gene has the attributes of tumor suppressors, sequence analysis showed no resemblance to other known genes in this family. Instead, the gene encodes three cytoskeletal-associated-protein-glycine-conserved (CAP-GLY) domains, which are found in proteins that coordinate the attachment of organelles to microtubules. CYLD also has sequence homology to the catalytic domain of ubiquitin carboxy-terminal hydrolases (UCH).
Bignell GR et al., "Identification of familial cylindromatosis tumour-suppressor gene." Nature Genetics 25 (2): 160-5, 2000.
High Anxiety (Back to Top)
Three papers in the April issue of Nature Genetics report that stress response in mice can be quelled by one of two known corticotropin-releasing hormone receptors (Crhr). Crorticotropin-releasing hormone is secreted by the hypothalamus in response to stress, and it, in turn, stimulates the pituitary gland to release additional hormones that stoke the stress response, like blood pressure elevating hormones and immune response suppressing hormones.
While previously two receptors had been found, Crhr1 and Crhr2, the exact function of each had not been known. In the new work, Kuo-Fen Lee (Salk Institute), Mary Stenzel-Poore (Oregon Health Sciences University), and Michael Rosenfeld (University of California, San Diego) and their colleagues found that male knock-out mice for the crhr2 gene demonstrated high anxiety and a greater stress response in stress-inducing situations like bright lights and heights. Furthermore, mice with one functional copy of the crhr2 gene showed anxiety levels intermediate between normal mice and double knock-outs. And feeding the mice a drug that selective blocks the crhr2 receptor also induced anxiety.
Similar experiments using drugs that inhibit specifically the crhr1 receptor failed to produce increased anxiety, ruling out the Crhr1 receptor as the mediator of this effect. And, in an intriguing finding, the researchers discovered that female crhr2-deficient mice did not show increased anxiety at all.
These findings confirm the importance of Crhr2 in mediating the response to stress, and will help clarify how different classes of hormones and receptors interact in the regulation of this important physiological state.
Kishimoto T. et al., "Deletion of crhr2 reveals an anxiolytic role for corticotropin-releasing hormone receptor 2," Nature Genetics 24(4): 410-14, 2000.
Bale TL et al., "Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress," Nature Genetics 24(4):410-4, 2000.
Coste, SC et al., "Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2," Nature Genetics 24(4):403-9, 2000.
