FDA's Top 5 Drug GMP Inspection Citations In FY2018 — With FDA Analysis

This is the second part of a two-part article counting down the FDA’s top 10 most-common drug GMP inspection citations for the agency’s 2018 fiscal year (FY2018). The list was presented by FDA Supervisory Consumer Safety Officer Dell Moller during a panel session at the FDA/Xavier PharmaLink conference at Xavier University earlier this year.
Part 1 covered citations #10 through #6, and this part covers citations #5 through #1. For each citation, its rank from FY2017, the related Code of Federal Regulations (CFR) Title 21 reference, and a brief FDA analysis from Moller’s presentation slides are included. Comments from Moller and fellow panelists Art Czabaniuk, FDA Office of Pharmaceutical Quality Operations (OPQO) Division 3 program director, and Lindsey Schwierjohann, OPQO Division 3 investigator, are also included (in italics).
Following the citations list is further discussion among the panelists on inspection trends, other inspection findings, and how FDA investigators get a sense of the quality culture of an organization.
#5 (FY2017 rank: not in the top 10) – Equipment and utensils are not cleaned or maintained or not sanitized at appropriate intervals to prevent contamination that would alter the safety, identity, strength, quality or purity of the drug product. [21 CFR 211.67(a)]
FDA Analysis: This parallels the equipment cleaning citation (#7) where rather than no/inadequate SOPs for cleaning, the utensils and/or equipment are cleaned but there is some type of left-over residue or solution on or in the equipment/utensil. This could be an indication of the preoperative inspections needing more time or better training/more expertise to conduct properly.
FDA Panelist Comments:
Dell Moller: It is the same thought process as #7 [see Part 1]: Are the cleaning and sanitation crew or the people responsible for doing this given enough time, enough preparation, and adequate training? And then the inspection crew walking in after that — are they given enough time and training, and do they have the expertise to do the inspection?
Make sure your equipment is clean and free from contaminants. Have QA do walk-throughs of your production areas and look at the equipment. Is it getting old and needs to be replaced? Is it being maintained correctly? Investigators only see a snapshot of your facility. If we find his, why didn’t the Quality department find it before the inspection?
Art Czabaniuk: When I have been out on inspections, I have actually seen apparent rust and dirt on equipment, and lubrication leaks. Anything that can alter the safety and purity of the drug will be tagged for this citation.
Moller (in response to an audience member asking if FDA expects all cleaning to be validated or verified as effective): If you are cleaning equipment to remove the previous product for the equipment to be used for another product or strength of the same product, the cleaning needs to remove the residues. That must be validated. And typically, we would also expect to see some verification after that each time it is performed.
You have to know if your cleaning is effective every time — not just the three or four times from your validation run.
Czabaniuk: Typically, what we see is a cleaning matrix that uses toxicity and difficulty to remove as the two parameters that cleaning procedures are designed against. New products are characterized against the matrix. The answer is “yes,” cleaning procedures need to be validated.
#4 (FY2017 rank: not in the top 10) – No written SOPs for production/process controls designed to assure the drug products meet their necessary attributes. [21 CFR 211.100(a)]
FDA Analysis: Lack of or an incomplete SOP. A properly written SOP provides sufficient information to assure personnel can perform each activity correctly and consistently and can achieve the expected outcome each time along with appropriate documentation to allow for Production, QA and regulatory review. This also includes what to do if something does not go as planned or does not go quite right.
FDA Panelist Comments:
Moller: We understand that things are going to go wrong. How you handle it when it goes wrong is what we care about.
Czabaniuk: Someone mentioned earlier simplifying procedures. I had direct experience with a firm whose procedures were too complex. When they got that feedback, they rewrote them so that more of them were work instructions — there was less narrative and more in the way of steps. It did, in fact, improve the outcome. Simplifying procedures and making them easier to read and understand for the users is a good piece of advice.
Moller: That is critical. If your folks don’t understand the procedures, they are going to gloss over them and assume they are trained; then they are going to look at the next person and do it the same way they are doing it. If that person is doing it right, he is good to go. If that person is not doing it right, now an incorrect template has been stamped on other employees, none of whom are doing it correctly.
Czabaniuk: I have been at firms where I have looked at gowning procedures and have seen nothing but words. But at other firms I have seen gowning procedures with a lot of pictures. I am a visual person, so being able to see the photos of how a person is supposed to gown each step of the way makes it easy to follow. An easy-to-follow SOP is the best type of SOP. You want all of your employees to be able to understand how to follow the procedure.
Moller: I was at a company doing an inspection some time ago and asked them for a couple of procedures. They had lumped them all into one procedure. It was so thick that when I put it into my bag to take it back to the hotel to review it, my shoulder dropped about an inch.
#3 (FY2017 rank: #3) – Failure to thoroughly review any/all unexplained discrepancy or failure of batch/components to meet their specs — whether or not the batch has been distributed. [21 CFR 211.192]
FDA Analysis: Incomplete or absent Investigations: The firm is responsible to assure that all deviations not only are properly investigated with an appropriate conclusion and thoroughly and are accurately documented, including all potentially affected batches assessed/evaluated, but also are appropriately dispositioned and documented as such.
FDA Panelist Comments:
Lindsey Schwierjohann: Look for trends. Ensure everything is documented.
Czabaniuk: You need to be sure the investigation is thorough, that all the documentation including batch records was reviewed, that root cause is determined, and that corrective actions are taken. An investigation that is not thorough is better than no investigation. Make sure everything is documented in detail.
#2 (FY2017 rank: #2) – Laboratory controls do not include the establishment of scientifically sound and appropriate specs/standards/sampling plans/methods designed to assure drug products conform to specs/standards… [21 CFR 211.160(b)]
FDA Analysis: The expectation is you rely on good science. So why is this still being observed? This includes laboratory investigations having a sound investigation strategy and not just retesting or testing into compliance. It also includes, if used, the proper application and use of the outlier test.
FDA Panelist Comments:
Moller: This is where a lot of people get hung up in their lab investigations.
Schwierjohann: I have gone through lab data and seen something in the audit trail or in the data and asked to see the OOS (out-of-specification result). The OOS is very nicely outlined, but then in the data I see other injections and other tests performed. When I have asked, I have been told it is “prelim testing” or “an investigation” or “testing some things before we go into the investigation.” Everything should be captured within the scope of the investigation. There shouldn’t be other testing prior to the investigation. Everything should be captured, reviewed, and reported to the Quality unit. If you have some initial testing that you do in Phase 1, for example, that is fine, but make sure it is documented and part of the scope. You should not be doing anything outside the investigation. You have SOPs that outline how to do testing when you have an OOS. Make sure you follow them, and it is all documented and justified and reviewed by the appropriate person.
Czabaniuk: FDA has a guidance document for investigating OOS test results, available online. You can find it and follow it for OOS investigations in the laboratory.
Moller: FDA investigators looking at OOS investigations are looking to see that a root cause was determined, what the corrective action was, and how the batch was dispositioned. Even if you reject a batch, you still need to perform an investigation.
The message seems to have gotten across that test injections are unacceptable. As creative and innovative as you all are, a quality check that uses product instead of a standard is a test injection.
Czabaniuk: The best practice I have observed is trending of OOS by product type and by analytical chemist to see if there are any issues outside the science or the chemistry. As an example of that, I recall a topical product that was frequently out of spec. That is a red flag for us. We were looking at a number of OOS results, and the company explained to us that the issue was the SOP — that the extraction efficiency is low for this step in the SOP. That was their root cause over a period of a year. We questioned whether it was properly validated and whether it was the correct procedure to be using. We suggested they look at other solvents for extraction and revisit the method. The message is if you are coming to the same reason over and over for why OOSs are recurring, you should take it one step further. If it isn’t working, adjust accordingly.
#1 (FY2017 rank: #1) – Responsibilities & Procedures for the QA Unit are not in writing or fully followed. [21 CFR 211.22(d)]
FDA Analysis: Does QA have the responsibility and authority to carry out its job effectively and efficiently? Are all of the responsibilities of the QA unit written and documented? Has top management “bought in” to this concept of having a robust quality system in place positively affecting the bottom line?
FDA Panelist Comments:
Moller: If QA has the authority, are they using it? Do they know what they are supposed to do? Has management bought in on the fact that when QA walks through production and they see an issue and bring it to someone’s attention, is it going to get handled? If QA sees an issue, it needs to be taken care of. If it cannot be taken care of at the time, it needs to be put on a list and be dealt with at some point.
Czabaniuk: This points to all the previous observations. Those things do not happen unless the Quality department is involved.
Schwierjohann: Throughout the inspection, we are evaluating the Quality group and asking, “Do you have a presence in your facility? Are you somebody that people can talk to?” It is important for Quality to have a good relationship with Production and understand their role. It needs to be clear that they are not out there to make things more difficult, but to impact the outcome and the total quality of the product.
Czabaniuk: Exactly. Quality should have its hands in everything performed at a pharmaceutical firm. Quality should be involved at every step of the process. Quality should always be looking at the risk at every phase of drug production. They should be doing walk-throughs, as Lindsey [Schwierjohann] said earlier, to make sure that Quality is involved at every step of the process, in every system. The patient comes first.
Other Common Findings
In addition to the top ten 483 citations for FY 2018, Moller also shared a slide with other common findings. They are listed below with Moller’s comments, if any, in italic.
Training: Personnel are either not trained or not given the appropriate training for the activities they are performing.
Equipment: Equipment is not: of suitable size or design; located appropriately; easily cleanable to facilitate operations.
Moller: I have seen equipment is being overutilized, pushed beyond its capabilities. There was a discussion about unit operations earlier in the week. Material went from a blender into a bin. The bin was rolled across the floor, halfway across the plant, possibly across rough flooring, then lifted and dumped into something else for mixing or compression. What does that do to the uniformity of the product?
Stability: Lack of appropriate stability procedures
Moller: For example, pulling stability samples out and not testing them for months or years.
Lab: Allowing the analyst or the reviewer the potential to either change data, delete data, or move data to a folder that is not reviewed or does not go through the QA process.
Moller: Deleting data is never a good thing. You can use it for historical trending. You can use it to see how often an event happens. This goes to the issue of data integrity, which we continue to see throughout our inspections.
Procedures: In general, procedures not being followed.
Little Has Changed Over Time
Moller shared a graphic showing the top observations for the last five fiscal years and, for comparison, FY06. The citations are color-coded, meaning the same color box is the same observation.
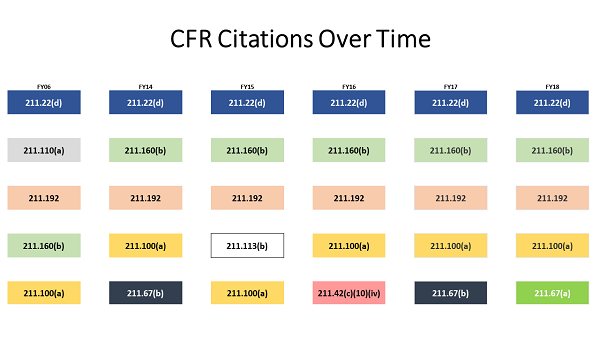
Moller: What we found were the same top 4 citations were frequently observed from year to year on inspections. The “specifically” or “for example” may be different, but the root citation for these observations often remains the same.
Evaluating Quality Culture
Moller: When we go into companies to do inspections, aside from looking at data, production lines, and movement of people and materials, there is an overall quality assessment going on. I am sure you have heard this before.
Moller then discussed the factors he looks for when assessing a company’s quality culture during an inspection. Here are some examples:
- Is there a quality mindset (from top to bottom) in all aspects of the operations, not just the Quality unit? Is the CEO — or the owner, if it is a smaller company — buying into the same thing the management and shop floor personnel are buying into?
- Are they doing the right thing because it is the right thing to do, not just because the FDA expects it, and not just in the documentation but in all operations?
- Does the inspection indicate that quality permeates throughout the company environment? Is it systemic? Is it apparent when we do our walk throughs? Is it in the atmosphere of the company?
- This doesn’t mean just putting up metric posters or having the slogan “Right First Time” or “There is no ‘I’ in Team.”
Moller: Signs do not make a quality environment. They are tools to get there. The company needs to be a team, all working together, hopefully getting things done right the first time.
Q&A Focuses on Quality Culture
During the Q&A after the presentation, an audience member asked for examples of things the FDA panelists have seen during inspection that gave them confidence that quality was systemic in the company they were inspecting.
Moller: I look for Production and Quality folks talking and having a good discussion. When those departments are comfortable with each other professionally, this is one indicator to me that quality is integral to the operations and doing the right thing.
Czabaniuk: My experience includes a couple of things. One is people doing a little bit more than they are required to. An example I gave earlier was trending OOS results. It is a good thing to drill down and find out where the issues are.
Another indicator is understanding the state of quality today. It is not unusual for our investigators to start an inspection and look at data to find out where the risks are and where the problems are. In a couple of days, they will be able to find out what the issues are and what the potential violations are. This is something that a good Quality organization does before FDA arrives — they identify those issues and take action. I know there is a lot of data to look at. But our investigators can find the issues in a few days.
Moller: Our field folks are trained to sort through data. If they can find it, you should be able to find it. Transparency between departments in the company is important. If Development and Tech Transfer and Production and Quality are all working together, that is another indication.
Editor's Note: For a similar review of the top 10 FDA 483 drug GMP observations for FY2017, click here.
The PharmaLink conference is one in a series of drug, medical device, artificial intelligence (AI), combination product, and EU Medical Device Regulation (EU MDR) conferences sponsored annually by Xavier Health. Read a review of how Xavier’s conferences are unique and engaging, written by Rob Wright, chief editor of Life Science Leader magazine, here.
About The Author:
 Jerry Chapman is a GMP professional with nearly 40 years of experience in the pharmaceutical industry. His experience includes numerous positions in development, manufacturing, and quality at the plant, site, and corporate levels. He designed and implemented a comprehensive “GMP Intelligence” process at Eli Lilly and again as a consultant at a top-five animal health firm. Chapman served as senior editor at International Pharmaceutical Quality for six years and is now senior GMP quality consultant at FDAzilla.
Jerry Chapman is a GMP professional with nearly 40 years of experience in the pharmaceutical industry. His experience includes numerous positions in development, manufacturing, and quality at the plant, site, and corporate levels. He designed and implemented a comprehensive “GMP Intelligence” process at Eli Lilly and again as a consultant at a top-five animal health firm. Chapman served as senior editor at International Pharmaceutical Quality for six years and is now senior GMP quality consultant at FDAzilla.
