FDA FY2020 Drug Inspection Observations And Trends
By Barbara Unger, Unger Consulting Inc.
A comprehensive GMP intelligence program includes monitoring health authority enforcement actions. The actions include FDA Form 483s, Establishment Inspection Reports, warning letters, recalls, import alerts, consent decree agreements, MHRA annual summaries of inspection deficiencies, and EU reports of GMDP noncompliance. This article presents the most recent publication of GMP drug inspection data, which address drug inspections conducted in FY2020. We examine data from FY2020 and evaluate five years' worth of trends in drug GMP inspection enforcement. For additional data on years before 2016, please refer to the article published last year.
include FDA Form 483s, Establishment Inspection Reports, warning letters, recalls, import alerts, consent decree agreements, MHRA annual summaries of inspection deficiencies, and EU reports of GMDP noncompliance. This article presents the most recent publication of GMP drug inspection data, which address drug inspections conducted in FY2020. We examine data from FY2020 and evaluate five years' worth of trends in drug GMP inspection enforcement. For additional data on years before 2016, please refer to the article published last year.
The presentation of some data herein differs from data presented on the FDA website, even though it uses the same raw data. For example, I combine all observation listings that cite 21 CFR 211.42(c) into a single value, rather than identifying them in separate line items with the exact same citation text. Likewise, I've consolidated §211.192 into a single item, rather than the five line items in the FDA’s data presentation.
The data do not represent the FDA's complete collection of inspection observations for the year. In past years, these data represented approximately one-third of all Form 483s issued, so conclusions must be tempered by the incomplete nature of the data. The FDA data include only Form 483s issued through its electronic system; it does not include Form 483s issued to API manufacturers because §211 is not applied to those manufacturers or Form 483s that are issued outside of the electronic system.
FDA Form 483 Inspection Observations
The striking feature for FY2020 is the number of Form 483s, which decreased to less than half of those issued in FY2019. This is shown below in Figure 1. FDA inspections came to a grinding halt early in the year with the travel and safety limitations based on the COVID-19 pandemic. This limitation will likely ensure that the number of inspections conducted and Form 483s issued continue to remain under the FY2019 values well into FY2021.

Figure 1: Total number of Form 438s in the system
Table 1 shows only the 15 most frequent inspection observation citations between FY2016 and FY 2020, while the tabulation on the FDA website shows all citations used in the fiscal year. The FDA uses the term "frequency" to represent the number of times the agency identified a specific citation in its tabulation. Table 1 presents those observations from the highest to lowest number for FY2020. Table 1 shows consistency in the years between FY2016 and FY2020 with respect to the identity of the most frequently cited regulations. The four most frequently cited regulations for FY2020 include:
- §211.192 Investigations of discrepancies moved from third place in FY2018 to second place in FY2019 and is in first place in FY2020. It has historically been among the most frequently cited regulations.
- §211.22(d) Procedures applicable to the quality control unit shall be in writing and shall be followed moved from first place in FY2019 to second place this year. Again, this is another regulation that has been among the top group for many years.
- §211.160(b) Lab controls should include scientifically sound specifications was fourth place last year and third place this year.
- §211.100(a) Production and process controls shall be supported by written procedures is a common observation issued to OTC manufacturers who frequently have not conducted process validation for some or all products.
New in the top group for FY2020 are §211.68(b) and §211.160(a). §211.68(b) likely represents the FDA's continued focus on data management and data integrity, particularly for electronic data both in manufacturing (e.g., electronic batch records) and laboratory instrumentation. §211.160(a) also fits into the group of data integrity regulations, requiring data to be saved at the time of performance, data must not be obscured or lost, and adequate procedures must be in place to control laboratory documentation.
Based on the limitations of having far fewer Form 483s in this year’s collection, it’s virtually impossible to identify year over year trends. Instead, I’ve taken a cumulative approach over the past five years, shown in Figure 2. The four most frequently cited regulations for these five years include:
- §211.192 Investigations of discrepancies moved from third place in FY2018 to second place in FY2019 and is in first place in FY2020. It has historically been among the most frequently cited regulations.
- §211.22(d) Procedures applicable to the quality unit shall be in writing and shall be followed moved from first place in FY2019 to second place this year. Again, this is another regulation that has been among the top group for many years.
- §211.160(b) Lab controls should include scientifically sound specifications was fourth place last year and third place this year.
- §211.42(c) Facilities shall include defined areas of sufficient size.
The top three in the group above are the same as the top three for FY202. Fourth place for this five-year aggregate period is 211.42(c) rather than 211.100(a), which was fourth place in FY2020.
The FDA’s focus on OTC manufacturers may explain some of the reasons that having and following procedures for the quality unit, §211.22(d), continues at or near the top of the list. Often, these firms often either do not have a quality unit or have one that fails to perform its responsibilities. The FDA continues to hold the quality unit responsible for implementing an effective quality system at all pharmaceutical firms. Also, OTC firms are often deficient in the validation of the manufacturing process, as captured in §211.100(a).
Table 1: Drug GMP Inspections, §211 Citation Frequency by Fiscal Year
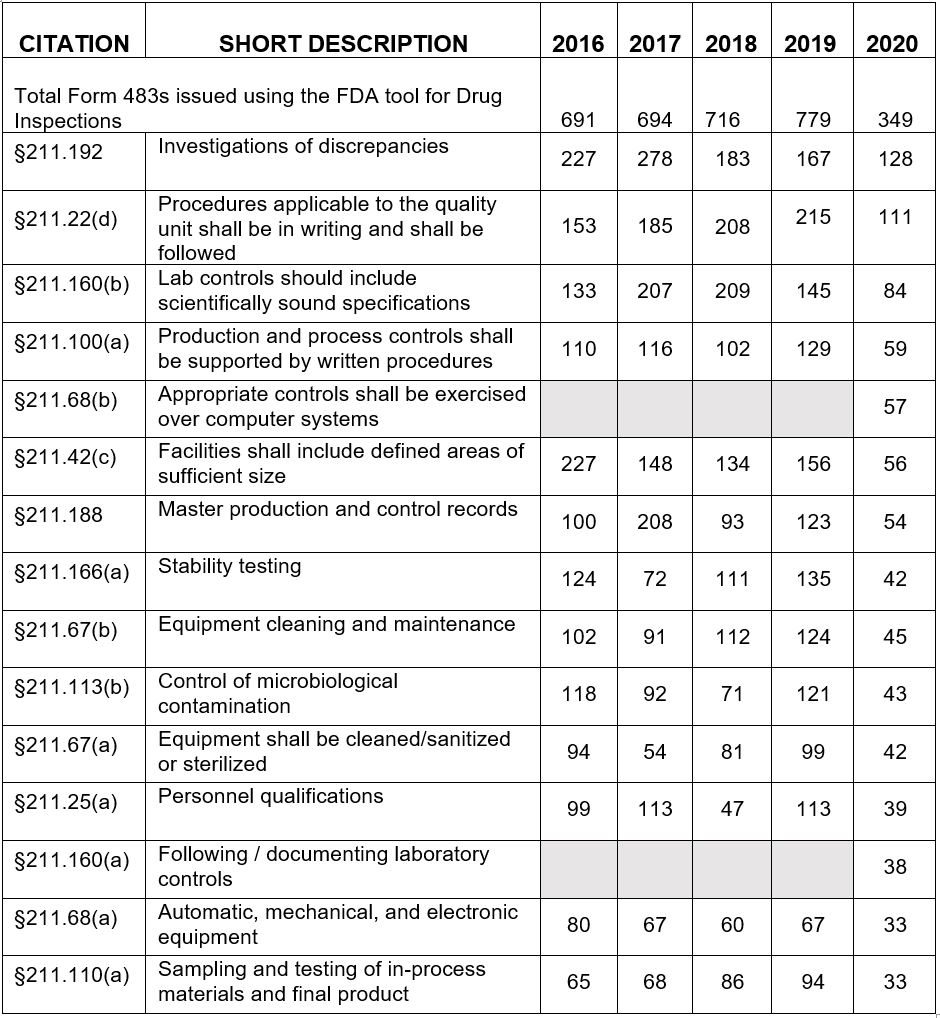

Figure 2: Top 10 Citations From 2016 Through 2020
Conclusions
For those who use inspection observations to monitor and improve their quality systems, the FDA's annual data provide ample resources against which firms can measure their potential vulnerabilities and gauge the probable focus areas during upcoming GMP inspections. The shuffling of positions among the most frequent observations likely reflects the FDA's recent focus on OTC manufacturers, where fundamental GMP understanding and compliance seems to be missing. Most of the observations at these sites reflect either a basic lack of understanding of FDA requirements or a conscious decision that compliance with these requirements would represent an unnecessary cost. Identifying and monitoring trends this year within the collection of 483 observations is difficult because of the substantial decrease in the number of inspections and Form 483s.
§211.192 moved up to first place this year after being in second place last year. It has consistently ranked among the most frequent citations; the industry still struggles with ensuring that investigations, including those for out of specification events, meet expectations. The lack of adequate written procedures and responsibilities for the quality unit, §211.22(d), remains a very consistent citation over the five years addressed herein. Form 483 observations that include text such as "The quality unit is inadequate…" often result in additional enforcement action, including warning letters.
I expect that even with effective vaccines available in the first half of CY2021, the number of on-site FDA inspections may remain limited through much of FY2021, continuing to provide challenges in identifying inspection trends. While the FDA is performing remote data reviews, it does not appear to count these as inspections and is not issuing forms 482 or 483, though this is all subject to change as we move forward. It would be reasonable to expect that the FDA will develop processes for the conduct of remote inspections or incorporate elements of remote inspections even when it returns to more frequent on-site inspections. The world has changed, and while it may take a while for the FDA to develop processes and procedures for this, we should expect that it will happen.
About The Author:
Barbara Unger formed Unger Consulting, Inc. to provide GMP auditing and regulatory intelligence services to the pharmaceutical industry, including general GMP auditing and auditing and remediation in the area of data management and data integrity. Her corporate auditing experience includes leadership of the Amgen corporate GMP audit group for APIs and quality systems. She also developed, implemented, and maintained the GMP regulatory intelligence program for eight years at Amgen. This included surveillance, analysis, and communication of GMP related legislation, regulations, guidance, and industry compliance enforcement trends. Unger was the first chairperson of the Rx-360 Monitoring and Reporting work group that summarized and published relevant GMP and supply chain related laws, regulations, and guidance. In addition, she was previously co-lead of the Rx-360 Data Integrity Working Group. You can contact her at bwunger123@gmail.com.
