Cryo-Shippers: Challenges, Controls, And Logistics
By Weiyang Li, Chris Masick, and Humberto Vega-Mercado, Bristol Myers Squibb

Cryo-shippers are a critical tool in today’s shipping operations involving frozen materials like embryonic cells, T cells, human tissues, and CAR T products. These devices are designed to maintain temperatures in cryogenic conditions (below minus 130°C) for extended periods while exposed to a variety of environmental conditions during storage and transportation that otherwise could damage the products when exposed to those conditions.
These shippers are passive systems that use a limited energy source such as liquid nitrogen (LN2) dry vapor to maintain the cryo temperature and are designed to be used multiple times. The cryo-shipper system typically consists of an inner container (dewar) that stores the liquid nitrogen and outer protective packaging for the dewar system. The dewar is a heavy-duty double-walled system, and the LN2 is stored in the absorbent materials in the walls of the dewar. As the LN2 evaporates, the LN2 vapor keeps the payload in the center of the dewar cold. This technology prevents the product from being submerged directly in the liquid phase of LN2.
Cryopreservation requires the formulated materials or products to be frozen at sub-zero conditions using controlled rate freezing to protect the cells while ensuring those cells remain viable (e.g., cell walls and cell content are not damaged) upon later thawing. The goal is to stop biological activity that will result in death and destruction of cells and cellular materials if maintained at standard refrigerated or room temperatures. Common products that are cryopreserved include, but are not limited to, purified embryonic cells, purified/concentrated T cells, intermediate products involving human tissues, CAR T products, and biologic products (lyo or suspensions).
Figure 1 presents an example where the use of cryopreservation and cryo-shippers is a key part of the manufacturing and handling of cryopreserved materials (e.g., T cells and CAR T cells). In this example, T cells collected from a donor (e.g., patient or healthy donor) are purified, concentrated, and cryopreserved with the goal of extending their shelf life while awaiting the final manufacture of CAR T products. Once the T-cells are genetically modified to express the chimeric antigen receptor (CAR) and expanded to a target number of cells, the CAR T cells are harvested, formulated, and cryopreserved. Shipping of the initially manufactured and preserved T cells using cryo-shippers may be required if these initial concentration and preservation steps are conducted in facilities dedicated to such manufacturing steps and separate from where the final drug product is produced. Similarly, once the drug product is ready for infusion into patients, cryo-shippers are used for transport of the product to the final infusion sites of care and treatment (e.g., hospitals).
Cryogenic temperatures can cause primary packaging materials to become fragile if they are stored and shipped below the glass transition temperature for a given material. The protection of fragile primary packaging materials can be solved by a combination of robust secondary packaging design and using protective materials inside the cryo-shipping system.
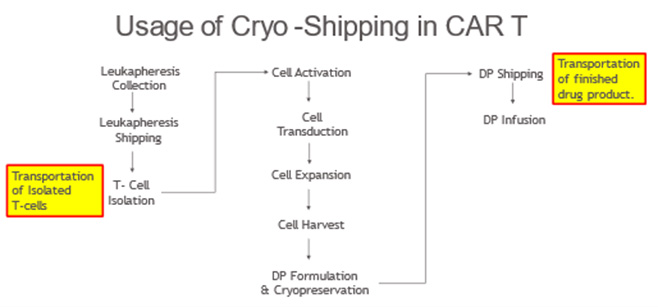
Figure 1. General process diagram for the manufacture of CAR T therapies.
Typical questions that are commonly asked regarding cryo-shippers include the following:
How far can a shipper travel and how long will it last at a predetermined temperature range?
The design of the cryo-shippers is focused on maintaining the cryogenic conditions required to preserve the frozen materials while in storage and transportation for extended periods (e.g., multiple days to weeks). These devices are used for shipping materials within intra-continental (e.g., within North America) as well as inter-continental boundaries (e.g., from North America to Europe). A key consideration is the proper handling of the devices during ground and air movements to ensure the integrity of the devices and protection of the products when exposed to various distinct shipping lanes.
What are the considerations for validation/qualification?
Qualification and validation activities for cryo-shippers start with clearly defining the user requirements specifications (URS). The URS should be based on product, packaging (e.g., cryovials, cryoboxes, etc.), and shipping lane-specific attributes and conditions (e.g., available ground and air modes of transportations, temperature requirements, environmental conditions, and handling considerations including expected amount of vibration and shock during transportation and handling). The URS will drive the design of qualification and validation activities. Those activities are summarized in Table 1.
Who are the providers of cryo-shippers and shipping services?
There are a variety of suppliers for cryo-shippers to the pharmaceutical industry. Table 2 presents some examples of shippers currently available in the market. An important element associated with these cryo-shippers is the availability and proper functionality of monitoring devices that offer real-time tracking of the shippers while in transit that includes information on shipper geolocation, internal and external temperature readings, tilt/shock/vibration events, light sensors to record potential openings, and battery level of the monitoring devices themselves.
Table 3 presents some examples of logistics providers involved in the handling of cryo-shipments. These providers will manage the delivery of empty, fully charged shippers, collection of shippers with packout materials and ready for transportation, tender to airline or train services, pick up from airline or train services, in-transit storage, and delivery to either storage location or treatment centers.
Table 1. Qualification and Validation Activities
| Activity | Description | Expectations |
| Design Qualification | Benchmark testing of shipping system and associated packaging components | Establish potential range of performance attributes (e.g., temperature range, hold duration, min/max payload). Finalize configuration of associated packaging components. |
| Operational Qualification | Laboratory studies of shipping system and associated packaging components using industry-standard test profiles (e.g., ISTA and ASTM standards) | Demonstrate shipping system can maintain predefined thermal and mechanical performance requirements under simulated worst-case stress conditions. |
| Performance Qualification | Real-world shipping studies using representative processes and shipping lanes | Demonstrate shipping system can maintain predefined thermal and mechanical performance requirements under actual shipment processes (e.g., packout, transport, receipt). |
Table 2. Example of cryo-shippers available across the industry (reference to products and service providers does not represent an endorsement by the authors or BMS)
| Cryo-Shipper | Description | Supplier |
| CRYOPORT Express – High Volume Dry Vapor Shipper | Liquid nitrogen is absorbed inside the dewar and released as dry vapor. The lid applied to the dewar reduces the loss of LN2 during transportation. Data logger and GPS tracking system are placed on top of the dewar lid. Metal shipping racks hold a variety of payload combinations inside shippers. |
CRYOPORT Systems |
| Biolife Solutions – DV10 DV4 | Liquid nitrogen is absorbed inside the dewar and released as dry vapor. The lid applied to the dewar reduces the loss of LN2 during transportation. Data logger and GPS tracking system are part of the dewar lid. Tyvek pouch holds a variety of payload combinations inside shippers. |
Biolife Solutions |
| Core Cryolab – MVE Shippers | Liquid nitrogen is absorbed inside the dewar. The lid applied to the dewar reduces the loss of LN2 during transportation. Vapor shippers with real-time GPS tracking and temperature loggers on the lid. Vapor shippers are available in various sizes. |
Core Cryolab Core Cryolab is a trademark of CooperSurgical Inc. |
Table 3. Example of cryo-shippers available across the industry (reference to products and service providers does not represent an endorsement by the authors or BMS)
| Logistic Providers | General Considerations | Expectations: | |||||||||||||||
| QuickStat, World Courier, Marken, DHL, Core Cryolab |
Available cryo-shipper model(s) and supplies | End users need to confirm correct shipper models to meet performance requirements, and couriers can maintain enough supplies on-hand to meet demands and ensure business continuity. | |||||||||||||||
| Location of courier warehouse and transit hubs | Multiple intra- and intercontinental warehousing and transit hubs available to ensure cryo-shippers can be stored, maintained, delivered to, and returned from various end user sites. | ||||||||||||||||
| Cryo-shipper annual usage rates | Tracking on the number of "turns" expected for different shipper models used in various shipping lanes and regions. Ensures renewal of supplies to remove overused systems which may result in failure of performance. | ||||||||||||||||
| Maintenance and refurbishment processes | Proper procedures are adhered to during initial charging of empty shippers to send to packout sites and inspection of returned shippers and equipment to ensure reusability over a set time period (e.g., annually or quarterly). Couriers should work in tandem with cryo-shipper manufacturers to develop and implement handling procedures and processes. | ||||||||||||||||
Table 4 summarizes the different elements necessary for proper selection of both shippers and logistics providers for handling and shipping of cryo-preserved materials.
Table 4. Elements for selection of shippers and logistic providers
| Element | Description | Considerations/Controls |
| Selection of Shipper | Evaluation of process needs vs. available options to ensure proper fit for use | Reliability, capacity, performance |
| Selection of Logistics Provider | Evaluation of capabilities to satisfy process demand and expectations | Reliability, customer services |
| Qualification of Shipping Lanes | Real-time shipping to assess overall performance in real-world conditions | Overall shipping time, temperature requirements, handling (ground/air) |
| Qualification of Shipper | Testing of worst-case shipping conditions to confirm shipper’s performance | Overall shipping time, temperature requirements, shock and vibration conditions, proper design of payload and associated packaging configurations |
| Active Monitoring | Real-time position tracking, monitoring of temperature, and shock/vibration | Access to portals where data can be monitored |
| Functional Requirements Spec. | Consolidation of final shipping system and associated packaging configuration | Consistency for sourcing, storage and handling requirements, part numbers. |
| Capacity – Content | Various payload configurations (vials, canes, cryobags, racks, etc.) depending on end user requirements. Primary packaging becomes brittle and fragile under cryogenic ultra-cold conditions. Use of protective secondary packaging/shipping accessories reduces impact of shock and minimizes freedom of motion within the dewar. |
It is essential to identify all potential configurations during USR development and use bracketing strategies during study design/development testing phase (DQ) to avoid repeat testing. Stress testing (ISTA/ASTM) various secondary packaging configurations during development testing phase (DQ/OQ) to ensure proper protection of primary packaging |
| Size and Weight | Shipping systems are often heavy (>75 lbs) with large outer dimensions and cumbersome to maneuver. Larger dimensions preclude use of smaller airplanes with limited cargo space. |
Use of built-in (outer casing with wheels) or accessory features (e.g., Cryoport Sliderite) to ease mobility challenges. Use of specialty couriers/white glove service providers to mitigate logistical challenges due to size/weight. |
| Temperature Control | Loss of temperature control possible under three conditions: 1. Prolonged placement on side orientation (LN2 dry vapor egresses at a faster rate). 2. Damages during transit (loss of vacuum insulation). Can be acute or due to prolonged usage. 3. Premature opening during transit (tamper or accidental). |
Potential controls: 1. Side orientation: active monitoring using built-in tilt sensors. 2. Damages: robust stress testing during validation, active monitoring using built-in shock sensors, ensuring robust post transit inspection process from service providers. 3. Premature opening: see tamper prevention. |
| Tamper Prevention | As noted above, tampering can lead to loss of temperature as well as chain-of-identity challenges placing internal product at risk. | Potential controls: 1. Built-in light and temperature sensors that can detect opening of the shipping system. 2. Accessory packaging (e.g., serialized security ties). 3. Use of specialty couriers emphasizing chain-of-custody practices. |
In conclusion, cryo-shippers are a useful tool to ensure materials and products requiring preservation and transportation at cryogenic conditions are properly maintained and protected. The selection of a cryo-shipper is not a trivial activity that must, at minimum, consider the following:
- Requirements (user and functional) must be clearly defined to ensure proper application.
- Procedures must be developed for the handling of the shippers prior, during, and after the cryopreserved material is ready for shipping.
- Qualification of the shippers must capture the expected environmental conditions along the shipping routes and stressors during the transportation activities.
- Supplier selection process (of both shipping system and logistical providers) is essential to ensure robust and successfully repeatable transportation process.
About the Authors:
 Weiyang Li is an associate director of cell therapy packaging technology at Bristol Myers Squibb where he focuses on packaging development and the qualification of temperature-controlled shipping systems for use in transportation of novel drug product and critical raw materials. He has a B.S. in packaging science from the Rochester Institute of Technology and a M.A. in philosophy from the State University of New York at Buffalo. Previously, he has worked in the packaging industry specializing in pharmaceutical and consumer products.
Weiyang Li is an associate director of cell therapy packaging technology at Bristol Myers Squibb where he focuses on packaging development and the qualification of temperature-controlled shipping systems for use in transportation of novel drug product and critical raw materials. He has a B.S. in packaging science from the Rochester Institute of Technology and a M.A. in philosophy from the State University of New York at Buffalo. Previously, he has worked in the packaging industry specializing in pharmaceutical and consumer products.
 Chris Masick is the technical director for global packaging of cell therapy at Bristol Myers Squibb, where he leads the teams responsible for designing and validating the packaging, labeling, and shipping systems for cell therapy around the globe. Chris has 20 years of packaging experience for multiple healthcare companies and has a B.S. in packaging science from Rochester Institute of Technology.
Chris Masick is the technical director for global packaging of cell therapy at Bristol Myers Squibb, where he leads the teams responsible for designing and validating the packaging, labeling, and shipping systems for cell therapy around the globe. Chris has 20 years of packaging experience for multiple healthcare companies and has a B.S. in packaging science from Rochester Institute of Technology.
 Humberto Vega, Ph.D., is executive director of BMS’ Cell Therapy Development, Global Manufacturing Sciences and Technology (GMS&T) and head of the Global Capabilities group based in Summit New Jersey. He has been in the pharmaceutical and food industries for over 35 years in multiple roles of increased responsibilities, including research assistant of food processing, process and manufacturing head of sterile and non-sterile pharmaceutical operations, senior scientist and associate director of validation, technology head of vaccine technology and engineering, associate director of MS&T, and senior director of MS&T. He holds B.S. and M.S. degrees in chemical engineering from the University of Puerto Rico and a Ph.D. in engineering science from Washington State University. He holds professional memberships in the Parenteral Drug Association (PDA), Institute of Food Technologists (IFT), and International Society for Pharmaceutical Engineering (ISPE).
Humberto Vega, Ph.D., is executive director of BMS’ Cell Therapy Development, Global Manufacturing Sciences and Technology (GMS&T) and head of the Global Capabilities group based in Summit New Jersey. He has been in the pharmaceutical and food industries for over 35 years in multiple roles of increased responsibilities, including research assistant of food processing, process and manufacturing head of sterile and non-sterile pharmaceutical operations, senior scientist and associate director of validation, technology head of vaccine technology and engineering, associate director of MS&T, and senior director of MS&T. He holds B.S. and M.S. degrees in chemical engineering from the University of Puerto Rico and a Ph.D. in engineering science from Washington State University. He holds professional memberships in the Parenteral Drug Association (PDA), Institute of Food Technologists (IFT), and International Society for Pharmaceutical Engineering (ISPE).
