Closed System Solutions
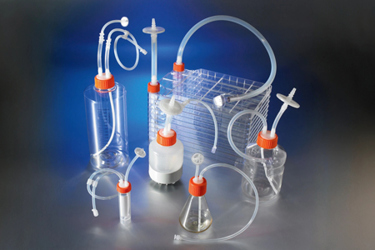
In laboratory and manufacturing environments, contamination risks can compromise the integrity of cell culture and aseptic liquid handling processes. Closed system solutions provide a reliable way to maintain sterility while improving efficiency and productivity. These single-use systems arrive sterile and ready to use, eliminating the need for in-house assembly and reducing the time and expense associated with sourcing components. With validated accessories such as filters, tubing, and connectors, these systems are customizable to meet specific workflow requirements.
Designed for sterility assurance and compliance with USP Class VI standards, these solutions are ideal for drug development and manufacturing applications. Whether you need pre-assembled vessels or aseptic transfer caps, these tools simplify workflows and support contamination-free processes.
Download the brochure below to explore how these solutions can optimize your aseptic workflow.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Bioprocess Online? Subscribe today.
