BME Researchers Discover How Superbugs Become Resistant To Antibiotics
By Mark Dwortzan
Many people with bacterial infections stop taking antibiotics when their symptoms improve, thereby allowing the hardy bacteria that survive to multiply and potentially mount a more powerful defense against future applications of the same drug. But a new study led by Professor James Collins (BME) indicates that sub-lethal doses of an antibiotic can trigger another, more alarming outcome, in which the targeted bacteria become cross-resistant to multiple antibiotics.
Collins, BME graduate student Michael Kohanski and post-doc Mark DePristo described their research in the Feb. 12 edition of Molecular Cell, findings that could spark considerable changes in how antibiotics are used across the globe as the public health community strives to combat the proliferation of multidrug-resistant strains of bacteria, the so-called "superbugs."
Two years ago, the researchers had proven that when applied in lethal doses, antibiotics stimulate the production of reactive oxygen species (ROS) molecules, or free radicals that damage DNA, protein and lipids in bacterial cells, contributing to their demise. In the Molecular Cell study, they demonstrated that the free radicals produced in targeted bacteria by a sub-lethal dose of an antibiotic live on to accelerate the formation of mutations that protect against a variety of antibiotics other than the administered drug.
"We know free radicals damage DNA, and when that happens, DNA repair systems get called into play that are known to introduce mistakes, or mutations, in the DNA," said Collins, who is also a Howard Hughes Medical Institute investigator. "We arrived at the hypothesis that sub-lethal levels of antibiotics could bump up the mutation rate via the production of free radicals, and lead to the dramatic emergence of multi-drug resistance."
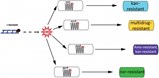
Testing their hypothesis on strains of the microbes E. coli and Staphylococcus, the researchers administered sub-lethal levels of five kinds of antibiotics to the targeted bacteria and showed that each boosted levels of ROS as well as mutations in the bacterial DNA. They next conducted a series of experiments to show that bacteria initially subjected to a sub-lethal dose of one of the antibiotics exhibited cross-resistance to a number of the other antibiotics — and in some cases, no resistance to the initially-applied antibiotic. Finally, they sequenced the bacterial genes known to cause resistance to each antibiotic and pinpointed mutations in those genes believed to be instrumental in protecting against that antibiotic.
"The sub-lethal levels dramatically drove up the mutation levels, and produced a wide array of mutations," Collins observed. "Because you're not killing with the antibiotics, you're allowing many different types of mutants to survive. We discovered that in this zoo of mutants, you can actually have a mutant that could be killed by the antibiotic that produced the mutation but, as a result of its mutation, be resistant to other antibiotics."
The group's findings underscore the potentially serious consequences to public health of administering antibiotics in low or incomplete doses. This is common practice among farmers who apply low levels of antibiotics to livestock feed; doctors who prescribe low levels of antibiotics as placebos for people with viral infections; and patients who don't follow the full course of antibiotic treatment.
"We need tighter regulations on the use of antibiotics, and doctors and patients need to be more disciplined in their prescription of antibiotics and patients need to be more disciplined in following their prescriptions," said Collins, whose research was funded by the National Institutes of Health and the Howard Hughes Medical Institute.
The study's findings may ultimately lead to the development of new antibiotic treatments enhanced with compounds designed to prevent the emergence of multi-drug resistance. For example, one potential treatment might inhibit the DNA damage repair systems that lead to the problematic mutations, while another might boost production of cell-destroying free radicals so that a low dose of antibiotic is sufficient to kill targeted bacterial cells.
SOURCE: BME
