Blood Plasma Fractionation: Engineering And Facility Design For The Future Of Biotherapeutics
By Sanjeev Kumar, NIRAS A/S, Denmark

In the rapidly evolving biopharmaceutical landscape, human blood plasma is a critical raw material for lifesaving therapies. Plasma-derived medicinal products (PDMPs) such as immunoglobulins, albumin, and clotting factors are essential for treating immunodeficiencies, bleeding disorders, and trauma. However, the journey from donor to drug involves a highly complex, regulated, and engineering-intensive process.
Here’s something you might not know: Over 90% of global plasma is processed in just a handful of countries. Developing local fractionation capacity is not only a healthcare priority but also a matter of national biosecurity.
As an engineering consultancy specializing in life sciences, we play a pivotal role in designing and optimizing plasma fractionation facilities that meet stringent regulatory standards while ensuring operational efficiency, scalability, and sustainability. This article explores the science, engineering, and infrastructure behind plasma fractionation and purification, with a focus on modern facility design and process integration.
From Battlefield Innovation To Biotech Breakthrough
The origins of plasma fractionation trace back to World War II, when the need for stable, transportable blood products led to the development of the Cohn cold ethanol fractionation process. Dr. Edwin Cohn’s method separated plasma proteins by manipulating ethanol concentration, pH, and temperature, enabling the isolation of therapeutically valuable fractions.
This innovation laid the foundation for today’s plasma-derived therapies and continues to influence modern bioprocessing strategies.
Clinical Relevance of Plasma-Derived Products
|
Product |
Primary Clinical Use |
|
Albumin |
Burns, shock, trauma, surgical blood loss |
|
IVIg |
Primary and secondary immunodeficiencies |
|
Factor VIII |
Hemophilia A |
|
Factor IX |
Hemophilia B |
|
PCC |
Multiple clotting factor deficiencies |
These biologics are not synthetically manufactured; they must be extracted from human plasma, making the engineering of fractionation facilities a matter of both public health and national resilience.
Engineering The Fractionation Process
1. Cohn Fractionation (cold ethanol precipitation)
The Cohn process remains the industry standard for initial plasma fractionation. It involves sequential protein precipitation by adjusting:
- Ethanol concentration: 8% to 40%
- Temperature: minus 3 degrees C to minus 6.5 degrees C
- pH: 4.8 to 7.3
Each step isolates a specific protein fraction:
- Fraction I: Fibrinogen
- Fraction II/III: Immunoglobulins
- Fraction IV: β and γ globulins (often discarded)
- Fraction V: Albumin
The process requires precise control of thermodynamic parameters and robust containment to handle flammable solvents.
2. Cryoprecipitation
This process targets Factor VIII and involves:
- controlled thawing of frozen plasma at ~0 degrees C
- separation of cryoprecipitate via centrifugation
- washing, freeze-drying, and formulation
Cryoprecipitate is rich in fibrinogen, von Willebrand factor, and Factor VIII, making it vital for hemophilia treatment.
3. Chromatography
Chromatographic methodologies, introduced into plasma fractionation workflows in the early 1980s, have significantly enhanced the recovery and purity of therapeutic plasma proteins compared to traditional ethanol-based fractionation methods such as the Cohn process.
These techniques exploit the physicochemical properties of plasma proteins — such as size, charge, and binding affinity — to achieve high-resolution separation under controlled conditions.
4. Hybrid Process (Cohn process and chromatography)
The hybrid plasma fractionation process combines the traditional Cohn cold ethanol fractionation with modern chromatographic purification techniques to enhance the quality, safety, and yield of plasma-derived products.
To meet modern purity and safety standards, hybrid processes integrate:
- tangential flow filtration (TFF) for buffer exchange and concentration
- chromatography for ion exchange and affinity for protein separation
- nanofiltration for viral clearance
- pasteurization and solvent/detergent treatment for Viral inactivation.
These steps are essential for producing sterile, high-purity biologics. This integrated approach enables higher purity, better viral safety, and greater process flexibility compliant with EMA and FDA guidelines, making it the preferred method in modern plasma fractionation facilities.
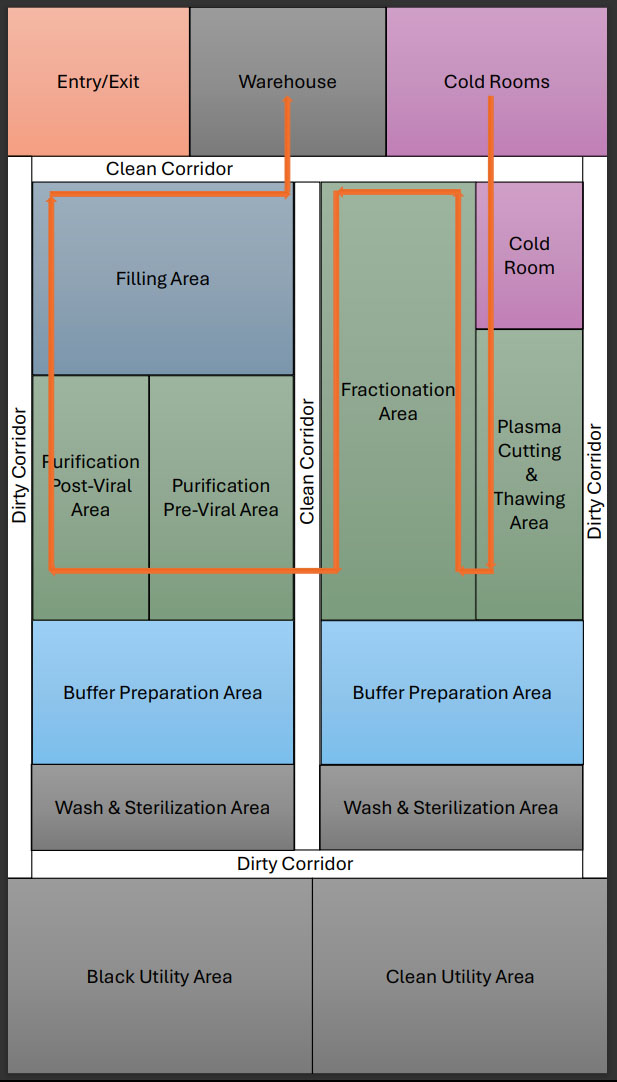
Facility Design: Engineering For GMP Compliance And Efficiency
Designing a plasma fractionation facility involves balancing regulatory compliance, process efficiency, and scalability. Key considerations include implementing:
- unidirectional personnel and material flow to prevent cross-contamination
- a clean and dirty corridor concept to control contamination and ensure product safety.
Modular vs. Stainless Steel Facilities
|
Design Type |
Advantages |
|
Conventional stainless steel |
Proven durability, suitable for large-scale operations, but water-intensive |
|
Single-use |
Rapid deployment, reduced cleaning validation, ideal for niche products |
|
Hybrid modular |
Combines flexibility and scalability; two- to three-year build timeline |
Choosing the right facility design — whether conventional stainless steel, single-use, or hybrid modular — is essential for optimizing production efficiency, scalability, and responsiveness to market demands. Aligning the design with your operational goals and product portfolio ensures long-term success and agility in a competitive biopharmaceutical landscape.
![]()
Cold Chain Infrastructure
Plasma must be stored at minus 30 degrees C throughout the supply chain, and cold rooms typically occupy 50% of warehouse space. Redundant refrigeration systems and temperature monitoring are critical.
Process Plant Core Area
|
Warehouse |
Fractionation |
Purification & Filling |
|
Plasma reception and screening |
Plasma bag/bottle cutting and thawing |
Pre-viral area |
|
Mini pooling and undertest storage |
Fractionation area: vessels, centrifuge, pump and filter press |
Post viral area |
|
Approved plasma storage |
Intermediate product storage |
Formualtion area |
|
Dispensing area |
|
Filling area |
Utilities And Environmental Controls
This includes:
- pure steam, WFI, and PW systems for cleaning and formulation
- HVAC systems for Grade B, C, and D background for critical areas
- glycol and black steam for process temperature maintenance
- ethanol storage and recovery systems with explosion-proof design.
Key Equipment And Process Integration
Engineering firms must specify and integrate equipment that meets GMP, biosafety, and automation requirements, including the following:
- Plasma Bag Openers: automated, sterile systems for high-throughput processing
- Fractionation Vessels: jacketed, temperature-controlled, with dual-flow agitators (anchor + pitch-blade), sterile tank vent filter
- Purification Vessels: jacketed, temperature-controlled, with bottom magnetic mixer, sterile tank vent filter
- Centrifuges: temperature-controlled chamber bowl separator for protein separation
- Filter Presses: plate and frame type for solid-liquid separation under aseptic conditions
- Pasturizer: a viral inactivation step to ensure the safety of the final product
- Freeze Dryers (Lyophilizers): for product stabilization
- Formulation and Fill/Finish Units: integrated with open RABS
All equipment must be CIP/SIP capable, validated, and integrated into DSC/MES systems for traceability.
Digitalization and Automation
Smart manufacturing is transforming plasma processing:
- DCS and MES: real-time monitoring, batch control, and electronic batch records
- Digital Twins: simulate process dynamics for optimization and scale-up
- Predictive Maintenance: reduces downtime and improves asset utilization.
These technologies enhance regulatory compliance, data integrity, and operational efficiency.
Sustainability And Resource Efficiency
As environmental regulations tighten, sustainable design is imperative. Sustainability is not only a regulatory requirement but also a driver of long-term cost efficiency. Key processes to achieve this include:
- ethanol recovery systems to reduce solvent waste
- energy-efficient refrigeration (cold storage) and HVAC systems
- water conservation in CIP cycles (more optimized process)
- waste minimization through process optimization and the three R technique (recycle, reuse, and reduce).
Global Industry Landscape
The plasma fractionation industry is led by a few multinational players:
- CSL Behring
- Takeda
- Grifols
- Octapharma
- Kedrion
- Sanquin
- China Biologic Products
- Bio Products Laboratory
- Biotest
- Intas Pharmaceuticals Ltd.
- Reliance Life Science Pvt. Ltd.
These companies operate state-of-the-art facilities with capacities ranging from 100,000 to over 1 million liters/year.
Market Outlook (2025–2030)
According to Fortune Business Insights, the 2025 plasma fractionation market size is estimated at $37+ billion, and it is projected to grow to $60 billion by 2030, a CAGR of about 9%. The top markets worldwide are the U.S., the EU5 (U.K., Germany, France, Spain, and Italy), China, and Japan.
Challenges To Overcome In The Production Of Plasma-Derived Therapeutics
- Supply Constraints: The supply is dependent on voluntary donations and is geographically concentrated.
- High Production Costs: Fractionation requires complex, multi-step processes with high validation overhead.
- Regulatory Burden: Extensive testing and documentation are required.
- Infectious Risk: Despite viral inactivation, residual risks remain.
- Cold Chain Dependence: Requires robust logistics and storage infrastructure.
- Ethical Concerns: Paid donations raise equity and safety issues.
- Rising Demand: Aging populations and rare disease diagnoses are increasing demand.
- Geopolitical Risk: There is global overreliance on a few countries for plasma collection.
Conclusion: Engineering For Impact
Blood plasma fractionation is a mission-critical domain where engineering excellence directly impacts patient outcomes. Fractionation facilities must be compliant, efficient, and future-ready to ensure the pharmaceutical industry can continue to offer lifesaving solutions.
About The Author:
 Sanjeev Kumar is a seasoned process engineer with NIRAS and has deep expertise in monoclonal antibody (mAb) production, blood plasma fractionation, and sterile injectable facility design. With a strong foundation in biopharmaceutical manufacturing and equipment lifecycle management, he excels in equipment design, operation, troubleshooting, and CQV execution and is known for his ability to coordinate across cross-functional teams and stakeholders. His hands-on experience with bioprocess systems and clean utility operations makes him a trusted contributor to greenfield, retrofit, and complex expansion projects in the biopharmaceutical industry.
Sanjeev Kumar is a seasoned process engineer with NIRAS and has deep expertise in monoclonal antibody (mAb) production, blood plasma fractionation, and sterile injectable facility design. With a strong foundation in biopharmaceutical manufacturing and equipment lifecycle management, he excels in equipment design, operation, troubleshooting, and CQV execution and is known for his ability to coordinate across cross-functional teams and stakeholders. His hands-on experience with bioprocess systems and clean utility operations makes him a trusted contributor to greenfield, retrofit, and complex expansion projects in the biopharmaceutical industry.
