Bioprocess Contaminant Detection Using Simple Western
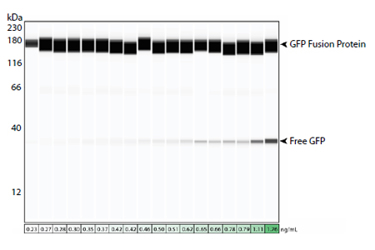
The identification and quantitation of process-related impurities during biotherapeutic development is critical to demonstrating the quality, efficacy and safety of the therapeutic agent. These impurities are typically residuals originating from the production cell line, the reagents and components used throughout cell culture/maintenance or during downstream processing. Residual proteins that go undetected can be recognized as foreign antigens in vivo, triggering a potentially fatal immune response. Additionally, traces of process-specific reagents or components may alter the product stability and reduce therapeutic efficacy. Therefore, the accurate detection of impurities is imperative but complicated, given the range of potential impurity sources or types, their presence in trace amounts and the complex matrix of the sample in which they exist.
In this application note, we focus on the accurate detection of four candidate contaminants that may be present during various stages of the therapeutic protein and vaccine development processes: host cell protein (HCP), Protein A, green fluorescence protein (GFP) and bovine serum albumin (BSA). Current methods for assessing the concentration of such residuals, like enzyme-linked immunosorbent assays (ELISAs), flow cytometry and traditional Western blotting are labor-intensive and prone to error. These traditional methods can also be misleading due to a lack of specificity and flexibility required to obtain a comprehensive contaminant profile and may exclude relevant critical information like size-based results, oligomerization state and low limit of detection. We’ll show you how highly sensitive and analytical Simple Western™ immunoassays deliver where others come up short.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Bioprocess Online? Subscribe today.
