Biopharma Facility Modular Design & Construction: Key Considerations
By BioPhorum
 As the pharmaceutical market expands into new modalities, manufacturers must move faster than ever before to manage multiple complex product pipelines. But extraordinary demand is straining biopharma companies’ ability to provide capacity. As a result, innovative solutions are needed to deliver and operate agile, flexible, and integrated facilities that seamlessly support these modalities from development through to commercialization.
As the pharmaceutical market expands into new modalities, manufacturers must move faster than ever before to manage multiple complex product pipelines. But extraordinary demand is straining biopharma companies’ ability to provide capacity. As a result, innovative solutions are needed to deliver and operate agile, flexible, and integrated facilities that seamlessly support these modalities from development through to commercialization.
The timeline for a biopharma production facility, from project inception to completion of qualification, is typically three to five years for a greenfield facility using traditional methods.
Modular construction can cut the schedule by 20%–50% and significantly lower costs, however it requires a design philosophy to match. This design philosophy is in fact the key enabler of a modular approach and subsequent standardizations, with benefits not only at the design stage but also in the fabrication, construction, qualification, and operation steps. It supports all construction methodologies, enabling biopharma companies to choose approaches that suit a particular location and region.
“Modular” can have different meanings to different individuals in the industry, depending on their background, expertise, and perspective. In terms of facility design, the term “modular design block” is used for “pre-engineered” or “predesigned” solutions. This approach suggests that a new facility design need not be unique but can use and leverage a pre-engineered set of component pieces. The further down the supply chain that standardized modules and components reach, the wider the benefits to the entire industry.
Can this same approach be applied to the fast-moving environment of new modalities in biologics? Does a modular approach work where manufacturing processes are less mature and the modalities are inherently more multi-product? The answer is yes.
The strategic imperative for new modalities in biopharmaceutical manufacturing is to deliver products through late-stage clinical development and build capacity rapidly to meet demand once products are approved. This implies that initial facilities will be followed by further facilities, either at the same process scale or a larger process scale. Biopharmaceutical process equipment is highly modularized, with very similar footprints for space, utilities, and operability. Irrespective of the manufacturer, this lends itself to the modular approach. Modules can be pre-engineered at several levels, from a work cell for a single process step to an entire building.
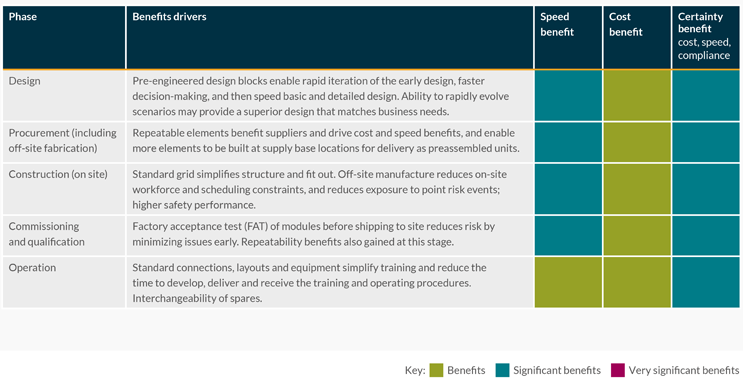
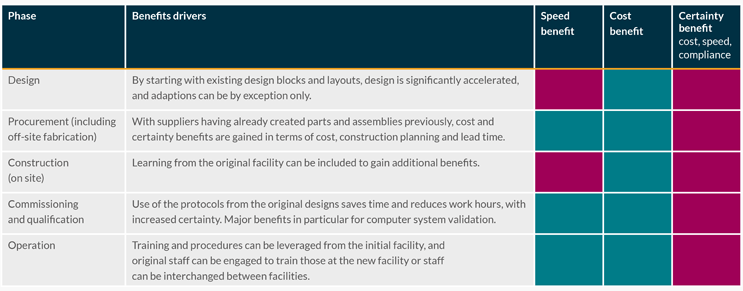
Tables 1 and 2 show the relative benefits of the modular design approach through a project life cycle for speed of delivery, cost of delivery, and certainty of schedule, cost, safety, or regulatory compliance.
Table 1: Benefits from the initial use of modular design
Table 2: Benefits from working with an existing library of engineered design blocks
A key concept to maximize this modular approach is that the physical footprint for each element must fit within a standard layout grid. Elements can be of different sizes, but each must be an exact combination of grid units.
How Is Modular Design Applied?
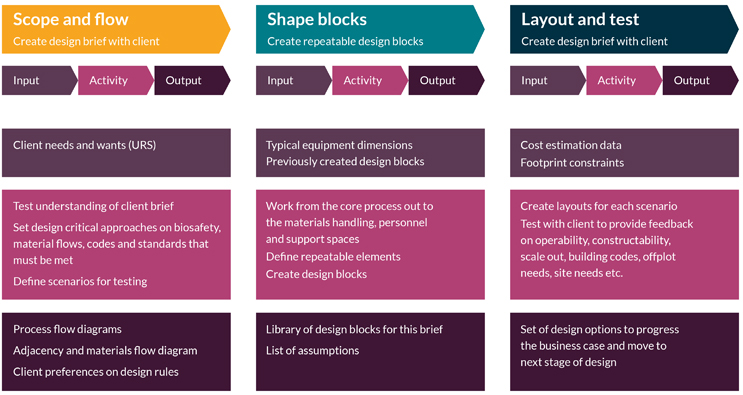
Modular design uses sequencing that is different from traditional design approaches, and there are parallels with agile project planning vs. waterfall planning. There are three main steps: scope and flow, shape the blocks, and layout and test. This design process overview is shown in Figure 1.
Figure 1: How modular design is applied
Having pre-engineered and grid-fitting modules does not preclude any of the three construction methodologies of traditional stick-built, modular hybrid, or fully modular (box-in-box). However, repeatable design elements drive standardization of parts and naturally lend themselves to modular fabrication. This gives the ability to manufacture and test a significant proportion of the facility off-site, while providing flexibility to the biopharma company and construction team to blend approaches according to local circumstances and preferences.
Modular Design And Capacity: A Strategy For Product Portfolio Management
Modular design helps to address biomanufacturer challenges by accelerating capacity additions, it also creates an opportunity for a significantly improved product portfolio management strategy. This is particularly relevant in the current market in which companies see increased competition, products with smaller patient populations, and portfolios with multiple therapy types.
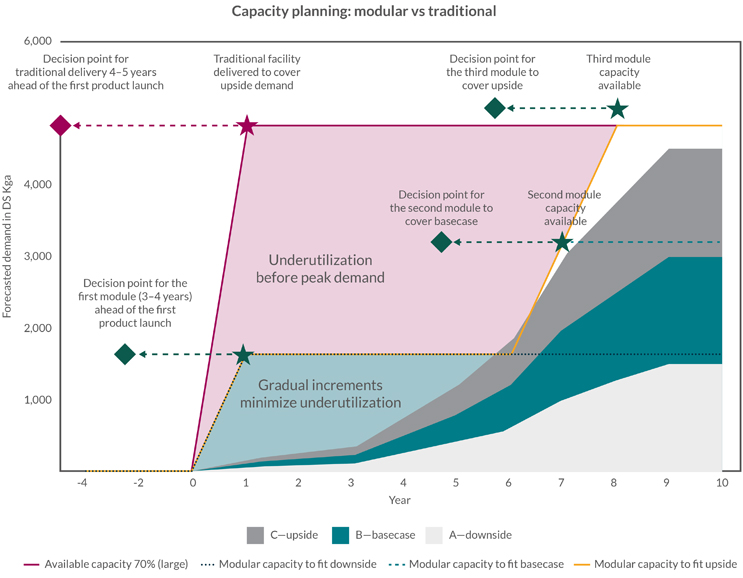
The one thing the industry can be certain of is that they will need to be able to react flexibly to changes in demand. The strategic flexing, addition, or repurposing of modular capacity enables rapid response to changes. Looking at the single product example, an answer to demand forecast volatility is to apply the modular logic to a forecasted aggregated demand curve for the portfolio. As illustrated in Figure 2, slicing the demand into standard “units of capacity” will allow investing in units of capacity to address the most likely demand for the mid-term (around three to five years).
Figure 2: Modular capacity vs. traditional single-phase investment
Based on modular design and modular capacity, this portfolio management strategy provides a road map for efficiently providing robust clinical and launch capacity using a scale-out approach.
Digital Enablers: Connecting Modular Design And Modular Construction
Modular design must be tightly integrated with modular construction, connecting individual stakeholder “links” into a robust value chain that provides the greatest amount of flexibility for facility owners while standardizing means and methods. This digitization of products and processes is highlighted as a key enabler for taking construction to the next level.
One obstacle is consistent data that are well-structured and usable by all project stakeholders throughout the project life cycle. The recent rapid acceleration of companies’ digital transformations has created an opportunity to capitalize on how technology can connect and accelerate modular design and modular construction.
Building information modeling (BIM) has become mainstream in many companies working in the architecture, engineering, and construction sectors, yet many still do not use it on all projects. Therefore, many owners, designers, contractors, and facility managers lack insight into the design and construction decisions being made by project stakeholders.
The current pressure on industry creates a strong strategic advantage for those who can start projects before process specifications are finalized, adapt to design and requirements changes throughout project delivery, etc. BIM and related digital enablers are at the core of creating the dynamic, data-driven digital transformation represented by the connected workflows of modular design and construction.
The benefits of achieving this level of digital transformation include outcomes-based design decisions supported by dynamic “what-if” optioneering for future facility expansion and reduction. Also, as BIM and related digital enablers are based on logical relationships between modules and blocks, not predetermined solutions, a modular framework remains site-agnostic, allowing for localization of a standard facility design across different locations and jurisdictions.
Aligning the biopharmaceutical industry around a modular facility design and construction framework will facilitate a quick, agile response to a dynamic technology and regulatory context. Also, promoting the creation, maintenance, and use of libraries for modular designs – at all scales and levels of standardization by all project stakeholders – enables significant reuse of facility elements between projects and ensures greater cost certainty and more rapid execution timelines.
Conclusion
By taking a modular design approach to biopharmaceutical production facilities, the design solution can be rapidly iterated and assessed against multiple relevant scenarios to aid decision-making and speed the justification and initiation of projects.
Following the rapid design phase, there are many more speed and cost benefits from repeatability through procurement, off-site fabrication and factory acceptance tests, simplifying on-site assembly and fit-out, and qualification and operation. This can take months or even years off a facility timeline and reduce schedule, cost, and regulatory approval risks. This can culminate in a phased approach to investment with major benefits to financial and business risk.
This article is a summary of a recent BioPhorum publication on the topic. To read more, check out the full report, Accelerating multi-product biopharmaceutical manufacturing facility project lifecycle through modular design (with a worked example of a late-stage clinical/early commercial viral vector facility).