ASTM International Pharmaceutical Cleaning Standards: Current Status And Future Direction
By ASTM International Cleaning Standards Team

Part of the Cleaning Validation For The 21st Century series
This article provides an update on the progress of the science-, risk-, and statistics-based ASTM International Cleaning Standards, which began with the publication of the ASTM E3106 Standard Guide for Science Based and Risk Based Cleaning Process Development and Validation1 under the E55 Committee (Manufacture of Pharmaceutical and Biopharmaceutical Products).1
Initially published in 2017, ASTM E3106 was based on the ICH Q9 Quality Risk Management guidance2 and FDA’s 2011 Process Validation guidance.3 ASTM E3106 provided high-level guidance on implementing a science-, risk-, and statistics-based approach to the development of cleaning processes, the selection of analytical methods, the qualification of cleaning processes and their ongoing monitoring.4
Since the publication of ASTM E3106, several companies have started to initiate its approaches, including GSK, which has successfully implemented these approaches in one of their vaccine facilities with a concomitant reduction in their cleaning efforts by approximately one-third.
Two primary principles of quality risk management found in ICH Q9 were fundamental in developing the ASTM E3106. These principles are:
- The evaluation of the risk to quality should be based on scientific knowledge and ultimately link to the protection of the patient.
- The level of effort, formality, and documentation of the quality risk management process should be commensurate with the level of risk.
It is important to recognize that ICH Q9 states that these two primary principles are applicable to validation (Annex II.6) and, more significantly, that they are applicable to cleaning, including setting acceptance limits for cleaning processes (Annex II.4).
So, we can reword the ICH Q9 principles to cover pharmaceutical cleaning as follows:
- “The evaluation of the risk in pharmaceutical cleaning should be based on scientific knowledge and ultimately link to the protection of the patient.
- The level of effort, formality, and documentation of the cleaning validation process should be commensurate with the level of risk."
Application of these ICH Q9 principles can provide a means to protect patient safety and also to determine effective and efficient cleaning validation activities that would focus efforts and resources where they provide the most value. So, the ASTM E3106 Standard’s Cleaning Risk Management Process was structured based on ICH Q9. An overview of the Cleaning Risk Management Process found in ASTM E3106 is shown in Figure 1.
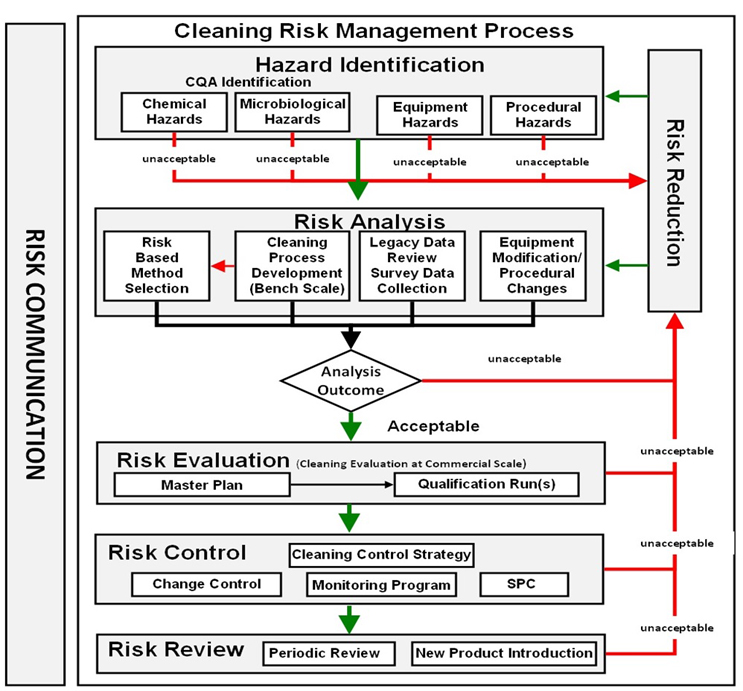
Figure 1: Overview of the ASTM E3106 Cleaning Risk Management Process. This diagram is a compilation of all the steps in the Cleaning Quality Risk Management Process in E3106.
Why ASTM International?
Readers may be curious as to why ASTM International was chosen for the development of these pharmaceutical cleaning standards. ASTM International is a not-for-profit organization formed in 1898, is one of the largest voluntary standards developing organizations in the world with over 12,500 global standards published through the efforts of over 30,000 volunteers. ASTM International provides a forum for the development and publication of international voluntary consensus standards for materials, products, systems, and services. ASTM International volunteers represent producers, users, consumers, governments, and academia from more than 140 countries.5
The development of ASTM International standards follows a very rigorous stepwise process that requires balloting and approval at the Sub-Committee stage, the Main Committee stage, and the full Society level and also includes a review at the ASTM International Committee on Standards before being published (Figure 3). ASTM International voting members can cast an affirmative, negative, or abstain vote. Negative votes must contain a reason for the negative vote. All comments received, whether in negative, affirmative, or abstain votes, must be addressed by the standard team before the ballot can move on to approval and publication. Ballots must achieve at least a 90% affirmative vote with at least 60% participation from the voting committee.
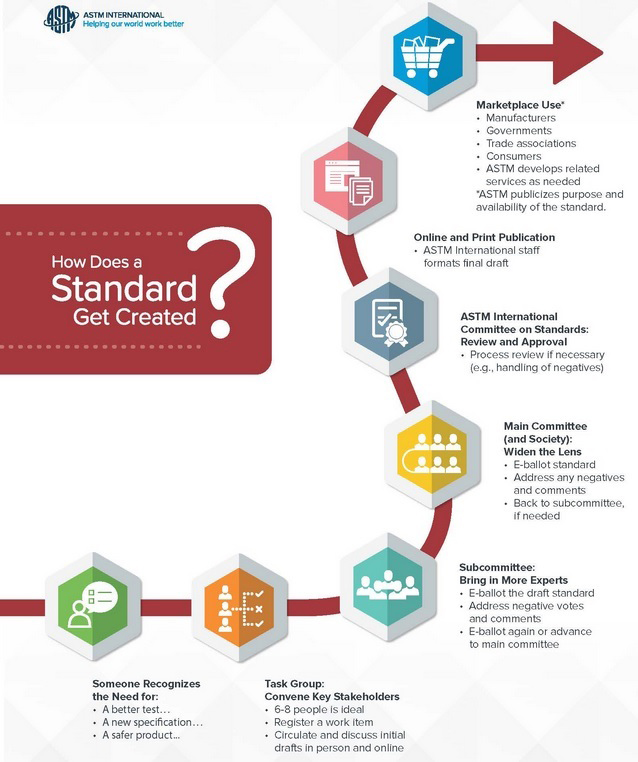
Figure 2: ASTM International Standards Development Process. Used with permission.
What is important about ASTM International being a voluntary consensus standard developing organization is that the National Technology Transfer and Advancement Act of 19956 directs federal agencies, such as the FDA, and departments to use technical standards developed by voluntary consensus bodies, unless impractical or inconsistent with applicable law. ASTM International was selected for the development of these pharmaceutical cleaning standards so that these standards could become recognized and followed by the FDA. In July of 2023, CDER released guidance on the start of its program to recognize such standards.7 In this guidance CDER states that ASTM International meets its criteria for a voluntary consensus standard developing organization. The ASTM International Cleaning Standards Team has currently submitted four standards for recognition in this program.
It is important to note that the ASTM E3106 and E3219 standards have been referenced in the World Health Organization (WHO) Technical Report 1033, Annex 2 “Points to consider when including Health-Based Exposure Limits (HBELs) in cleaning validation,”19 which attests to global regulatory recognition of their practical science and risk-based approach.
Published ASTM International Standards
While the ASTM E3106 presented a broad overview of how to implement the ICH Q9 Quality Risk Management process for pharmaceutical and medical device cleaning, most of the science-, risk-, and statistics-based approaches needed further detail and explanation. Since the publication of ASTM E3106, five additional standards have been created and published to support the implementation of the approach of E3106. These are briefly described below with references to published articles on each standard.
- E3219 “Standard Guide for Derivation of Health Based Exposure Limits (HBELs)”8 provides a detailed introduction to the approaches for utilizing preclinical and clinical data to derive health based exposure limits that toxicologists have been using over the past several decades. The E3219 is 30 pages in length, includes example HBEL calculations and a template for HBEL monographs, and has 148 references. The procedures outlined in this standard are expected to greatly aid pharmaceutical and medical device professionals in the derivation of acceptable levels of carryover and should be used in the risk assessment of cleaning processes using the ASTM E3106 Standard Guide for Science Based and Risk Based Cleaning Process Development and Validation.
- G121 “Standard Practice for Preparation of Contaminated Test Coupons for the Evaluation of Cleaning Agents”9 describes the procedure for the preparation of single- and double-sided contaminated test coupons for the evaluation of cleaning agents. Initially written for the cleaning of oxygen service equipment, it is now applicable to the evaluation of cleaning agents/processes for pharmaceutical manufacturing equipment and medical devices after manufacture. It also is applicable to other systems where chemical contamination is a concern.
- G122 “Standard Method for Evaluating the Effectiveness of Cleaning Agents and Processes”9 covers a procedure for evaluating the effectiveness and capability of cleaning agents to remove contaminants to the desired level. G122, also initially written for the cleaning of oxygen service equipment, now includes removing drug residues from manufacturing equipment and residues from medical devices (ASTM E3106).
- E3263 “Standard Practice for Qualification of Visual Inspection of Pharmaceutical Manufacturing Equipment and Medical Devices for Residues”10 was written, in part, to provide the necessary guidance for establishing qualified visual inspection programs to comply with the regulatory expectations concerning visual inspection found in the European Medicines Agency (EMA) Q&A on Health Based Exposure Limits.11 Shortly after the initial publication of ASTM E3263, an MHRA inspector requested the ASTM International Cleaning Team to make some revisions to make it more acceptable to Pharmaceutical Inspection Co-operation Scheme PIC/S12 inspectors. The E3463 was updated in 2022 with input from the Medicines and Healthcare Products Regulatory Agency (MHRA), FDA, and WHO.13
- E3418 “Standard Practice for Calculating Scientifically Justifiable Limits of Residues for Cleaning of Pharmaceutical and Medical Device Manufacturing Equipment and for Medical Devices” is the first comprehensive guide to setting limits for use in cleaning validation that includes all types of chemical residues, bioburden residues, endotoxin residues, and visual residues, with example calculations for swab and rinse sample limits for pharmaceutical and medical devices applications.14
ASTM International Standards In Progress
A number of proposed standards have been initiated as Work Items in ASTM International.
- WK78595 “Standard Guide for the Design of Clean in Place-Friendly Equipment for Pharmaceutical and Biopharmaceutical Applications (CbD Clean by Design).” This Work Item describes a practical implementation framework for the establishment of multidisciplinary teams to develop cleanability requirements and the subsequent specification, design, fabrication, and verification testing of manufacturing systems that are rapidly and robustly cleanable by automated CIP (clean in place), with reduced consumption of water, energy, and chemicals. The lessons in this guide could be extrapolated to manual and COP (clean out of place) processes as required or deemed appropriate. This item has been balloted and currently the comments from the ballot are being resolved.
- WK85802 “Standard Guide for Using FMECA and HACCP for Performing Risk Identification, Risk Analysis & Risk Control on Cleaning Processes for Pharmaceuticals and Medical Devices.” This standard provides guidance on how to use FMECAs and HACCPs to identify risk reduction and risk control strategies for cleaning processes. This standard was developed using an article on setting criticality levels in FMECAs (failure modes, effects, and criticality analysis) previously written by the ASTM International Cleaning Team members15. This article was used as a reference for the standard. This Work Item has been balloted at the E55 Main Committee and the Team is addressing the final comments received.
- WK86188 “Standard Guide for Risk Assessment of Cleaning Validation Programs.” This Work Item provides guidance on performing risk analysis on cleaning processes and cleaning validation program using process hazard analysis (PHA). The risk management tool is intended to be used in the introduction of new products, as well as risk assessments of existing cleaning validation programs. This Work Item has been drafted and is currently in team review.
- WK92658 “Standard Guide for Evaluating the Risk of Cleaning Processes Using Process Capability.” This standard is being developed from three articles on process capability previously written by some of the ASTM International Cleaning Team members.16-18 These articles will be used as references for the standard. This Work Item has just started.
- WK92599 “Standard Guide for Using Total Organic Carbon Analysis in Cleaning Validation.” This article is being written to introduce the concepts around using TOC in cleaning validation for pharmaceutical and medical device manufacturing. This Work Item has just started.
- WK92659 “Standard Practice Guide for Performing Swab and Rinse Recovery Studies in Cleaning Validation.” This Work Item has been created but no work on it has started yet.
Planned ASTM International Standards
There are also a number of standards that are planned to be undertaken in the near future.
- “Standard Practice for Statistics-based Sampling of Swab and Rinse Samples”
- “Standard Practice for Statistical Process Control for Cleaning Verification Data”
- “Standard Guide for Science and Risk Based Introduction of New Products”
Figure 3 graphically illustrates all of these published standards, current Work Items, and planned standards and where they fit into the ASTM E3106 Cleaning Quality Risk Management Process shown in Figure 1.
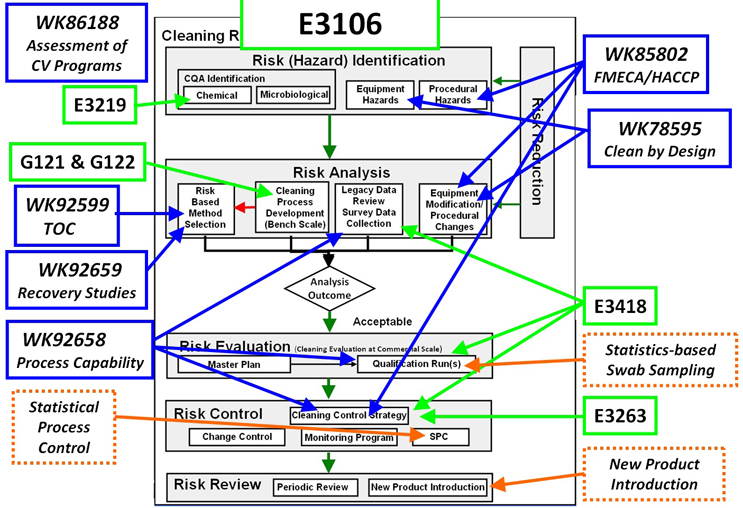
Figure 3: Status of ASTM INTERNATIONAL Standards in Support of ASTM E3106
----- (Published)
----- (In Progress)
----- (Planned)
Participation On ASTM International Standard Teams
Work on ASTM International Cleaning Standards began in 2014 with the development and publication of ASTM E3106. This effort expanded to include the five additional ASTM International standards being published along with the six ASTM Work Items started as discussed above. This effort also has grown and expanded to include more team members, collaborations with other ASTM International Committees (G04-Oxygen Service and F04-Medical Devices), and the addition of two new Team Leaders. Table 1 (below) shows the current active ASTM International Cleaning Team members and the ASTM International Committees they are participating in.
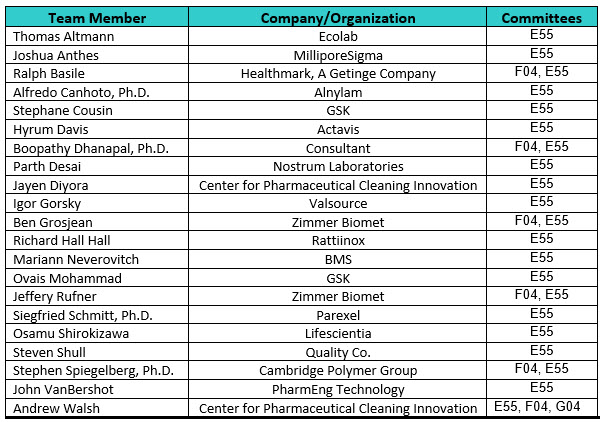
Table 1: ASTM International E55 Cleaning Standard Team Members
Table 2 (below) shows the ASTM International Cleaning Team Leaders and the Work Items they are currently leading.
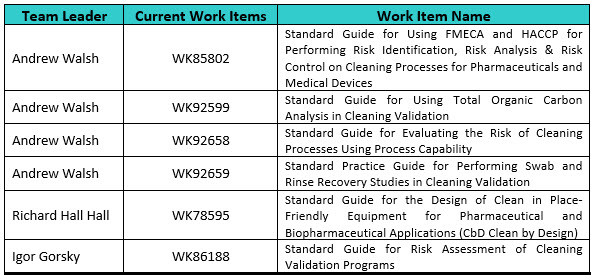
Table 2: ASTM International E55 Cleaning Work Items in Progress
The ASTM International Cleaning Team Leaders, Andrew Walsh, Igor Gorsky, and Richard Hall Hall have observed that industry interest in these ASTM standards has been rapidly growing and also have recognized that this effort could benefit from additional team members and Team Leaders. In 2023, it was announced on the ASTM E55 Pharmaceutical Standards LinkedIn discussion group that the ASTM International Cleaning Team has started an “open door” policy for the team meetings. The ASTM International Cleaning Team suggests that readers interested in what is happening with ASTM International Standards join the LinkedIn discussion groups, where they are free to post questions and comments concerning these standards.
If readers are interested in joining a team and working on a standard, or starting a new standard, the ASTM International Cleaning Team Leaders, Andrew Walsh (andywalsh@clean6sigma.com), Igor Gorsky (igorsky@valsource.com), and Richard Hall Hall (richard@rattiinox.com) suggest that they contact the Team Leaders directly.
Besides getting involved with ASTM International Cleaning Team, joining ASTM International is highly recommended as membership allows members to review and vote on proposed pharmaceutical and medical device standards when they are being balloted. While gaining voting rights is important, ASTM International membership also allows you to select one free volume of its Book of Standards. We highly recommend joining ASTM International and selecting Volume 14, which contains all of the current E55 Pharmaceutical standards as well as the ASTM Statistical Standards.
References
- American Society for Testing and Materials (ASTM) E3106-22 "Standard Guide for Science-Based and Risk-Based Cleaning Process Development and Validation" www.astm.org.
- International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, ICH Harmonized Tripartite Guideline, Quality Risk Management – Q9(R1), Final version Adopted on 18 January 2023
- FDA Guidance for Industry: Process Validation - General Principles and Practices January 2011, U.S. Food and Drug Administration (FDA), www.fda.gov.
- Walsh, Andrew, Thomas Altmann, Joel Bercu, Ph.D., Alfredo Canhoto, Ph.D., David G. Dolan Ph.D., Andreas Flueckiger, MD, Igor Gorsky, Jessica Graham, Ph.D., Ester Lovsin Barle, Ph.D., Ovais Mohammad, Mariann Neverovitch, and Osamu Shirokizawa "Introduction to the ASTM E3106 "Standard Guide to Science Based and Risk Based Cleaning Process Development and Validation" Pharmaceutical Online, June 2020
- www.astm.org
- https://www.congress.gov/bill/104th-congress/house-bill/2196
- “CDER’s Program for the Recognition of Voluntary Consensus Standards Related to Pharmaceutical Quality, Guidance for Industry”: https://www.fda.gov/media/121305/download
- Walsh, Andrew, Thomas Altmann, Joel Bercu, Ph.D., Alfredo Canhoto, Ph.D., David G. Dolan Ph.D., Andreas Flueckiger, MD, Igor Gorsky, Jessica Graham, Ph.D., Ester Lovsin Barle, Ph.D., Ovais Mohammad, Mariann Neverovitch, and Osamu Shirokizawa “Introduction To The ASTM E3219 Standard Guide For Derivation Of Health Based Exposure Limits (HBELs)” Pharmaceutical Online, July 2020
- Andrew Walsh and Ruijin Song “ASTM Standards For Cleanability Testing Of Pharmaceuticals And Medical Devices” Pharmaceutical Online, September 2020
- https://picscheme.org/en/picscheme
- Walsh, Andrew, Ralph Basile, Stéphane Cousin, Mariann Neverovitch, Mohammad Ovais, and Osamu Shirokizawa, “Introduction To ASTM E3263-20: Standard Practice For Qualification Of Visual Inspection Of Pharmaceutical Manufacturing Equipment And Medical Devices For Residues” Pharmaceutical Online, January 2021
- European Medicines Agency: Questions and answers on implementation of risk-based prevention of cross-contamination in production and “Guideline on setting health-based exposure limits for use in risk identification in the manufacture of different medicinal products in shared facilities," 19 April 2018 EMA/CHMP/CVMP/SWP/246844/2018
- Andrew Walsh, Thomas Altmann, Ralph Basile, Stéphane Cousin, Delane Dale, Parth Desai, Boopathy Dhanapal, Jayen Diyora, Christophe Gamblin, Igor Gorsky, Jove Graham, Reto Luginbuehl, Spiro Megremis, Ovais Mohammad, Mariann Neverovitch, Laurence O'Leary, Rod Parker, Siegfried Schmitt Ph.D., Osamu Shirokizawa, Stephen Spiegelberg, Ph.D., and Norma Turner “Revision Of The ASTM E3263 Standard For Visual Inspection Of Pharmaceutical Manufacturing Equipment And Medical Devices For Residues” Pharmaceutical Online, January 2023
- Andrew Walsh, Thomas Altmann, Ralph Basile, Alfredo Canhoto, Ph.D., Stéphane Cousin, Delane Dale, Parth Desai, Boopathy Dhanapal, Ph.D., Jayen Diyora, Christophe Gamblin, Igor Gorsky, Jove Graham, Benjamin Grosjean, Reto Luginbuehl, Spiro Megremis, Ovais Mohammad, Mariann Neverovitch, Rod Parker, Jeffrey Rufner, Siegfried Schmitt, Ph.D., Osamu Shirokizawa, Stephen Spiegelberg, Ph.D., and Randall Thoma, Ph.D. “Introduction To The New ASTM E3418, Standard Practice for Calculating Scientifically Justifiable Limits of Residues for Cleaning of Pharmaceutical and Medical Device Manufacturing Equipment and for Medical Devices” Pharmaceutical Online, December 2023
- Andrew Walsh; Thomas Altmann; Joshua Anthes; Ralph Basile; Alfredo Canhoto, Ph.D.; Stéphane Cousin; Delane Dale; Parth Desai; Boopathy Dhanapal, Ph.D.; Jayen Diyora; Christophe Gamblin; Igor Gorsky; Jove Graham; Benjamin Grosjean; Reto Luginbuehl; Spiro Megremis; Ovais Mohammad; Mariann Neverovitch; Rod Parker; Jeffrey Rufner; Siegfried Schmitt, Ph.D.; Osamu Shirokizawa; and Stephen Spiegelberg, Ph.D. “A Data-Derived Approach For Selecting Criticality Levels In FMECAs For Cleaning Process Risk Analysis” Pharmaceutical Online, February 2024
- Walsh, Andrew, Miquel Romero Obon and Ovais Mohammad "Calculating The Process Capabilities Of Cleaning Processes: A Primer" Pharmaceutical Online, November 2021
- Walsh, Andrew, Miquel Romero Obon and Ovais Mohammad "Calculating Process Capability Of Cleaning Processes With Partially Censored Data" Pharmaceutical Online, May 2022.
- Walsh, Andrew, Miquel Romero Obon, and Ovais Mohammad "Calculating Process Capability Of Cleaning Processes With Completely Censored Data" Pharmaceutical Online, October 2022
- World Health Organization (WHO) Technical Report 1033, Annex 2, Points to consider when including Health-Based Exposure Limits (HBELs) in cleaning validation, 201 https://www.who.int/publications/m/item/annex-2-trs-103
