ARIAD Reports Long-term Production of EPO by its Regulated Gene Expression Technology

ARIAD Regulated Gene Expression Technology (ARGENT)
Prolonged EPO Production
The Patents
At the Annual Gene Therapy Symposium at Keystone Colorado, researchers from ARIAD Pharmaceuticals Inc. (Cambridge, MA) presented new findings obtained with the company's proprietary gene regulation and gene activation technologies. On January 10, Victor M. Rivera, project manager for regulated gene therapy at ARIAD, presented details of preclinical studies on the delivery of therapeutic proteins, such as erythropoietin (EPO) and insulin, using gene therapy. Also presented was the development of new and proprietary components of the ARIAD Regulated Gene Expression Technology (ARGENT) system, which significantly improve the effectiveness of protein delivery.
On January 11, ARIAD announced that the company has been issued two patents covering pharmaceutical regulation of fundamental cellular processes through drug-mediated dimerization of proteins. Dimerization, or clustering of molecules within the cell, is the key step in many cellular processes, including gene expression, protein secretion, cell growth, and cell death. The claims in the two recently issued patents (U.S. Patent Nos. 6,011,018 and 5,994,313) cover methods for expression of important therapeutic proteins and induction of apoptosis (cell death), respectively.
ARIAD Regulated Gene Expression Technology (ARGENT) (Back to Top)
ARGENT is based on the principle that intracellular protein function can be regulated by highly specific protein interactions. Researchers at ARIAD have designed intracellular transcription factors, whose interactions are dependent upon small-molecule drugs. When the engineered cells are treated with the appropriate regulatory drug, the drug causes dimerization of the transcription factors, resulting in activation of a biological response in the engineered cells (Figure 1). Thus, this technology is designed to allow complex biological responses to be brought under direct pharmacologic control.

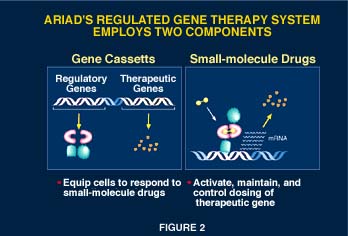
Implementation of ARGENT requires a two-component system (Figure 2). The first component, the gene cassette, is introduced into the patient. In addition to a therapeutic gene, the gene cassette contains regulatory genes that encode the engineered transcription factors that control gene expression. The second component of the system is the small-molecule drug that controls the activity of the engineered transcription factors, thus activating the therapeutic protein-producing mechanisms in engineered cells in a dose-dependent manner.
By using ARGENT-based gene activators, protein delivery can be controlled by an orally active small-molecule drug. Effective therapy depends on producing sufficient quantities of the protein, which, in turn, is limited by the potency of the gene activator protein. ARIAD scientists have discovered novel gene activators that are the most potent described to date and have devised methods to further take advantage of their potency by "bundling" a cluster of gene activator proteins together instead of relying on an individual molecule. The new ARGENT components provide dramatic increases in the expression of genes under conditions of clinical gene therapy. ARIAD scientists have incorporated this new technology into gene delivery vectors for testing in animal models. The bundling technology was described in a recent issue of The Proceedings of the National Academy of Sciences (S. Natesan et al. (1999) A general strategy to enhance the potency of chimeric transcriptional activators. 96, 13898-13903).
Prolonged EPO Production (Back to Top)
Rivera presented the latest results on the use of ARGENT gene therapy to deliver erythropoietin (EPO) in rhesus monkeys, done in collaboration with the Institute for Human Gene Therapy (IHGT) at the University of Pennsylvania (Philadelphia), led by James M. Wilson. As reported last year in Science, a regulated EPO gene was introduced into rhesus monkeys using an adeno-associated, non-pathogenic viral (AAV) vector administered intramuscularly. Administration of the orally active drug, rapamycin, stimulated EPO production and increased the number of red blood cells. In continuing studies, ARIAD and IHGT scientists have demonstrated that EPO production can be repeatedly stimulated with rapamycin in several monkeys for a period of over 450 days. The new data demonstrate the feasibility of long-term delivery of EPO by a one-time administration of gene delivery vector followed by use of an oral drug to induce protein production.
"Development of the novel ARGENT components should further broaden the applicability of our regulated gene therapy system by allowing higher blood levels of proteins to be produced," said Harvey J. Berger, chairman and CEO of ARIAD. "In addition, the latest data on the ARGENT system in rhesus monkeys emphasize the broad potential of ARGENT for long-term delivery of many secreted proteins. ARGENT-based therapeutics should markedly improve the safety and efficacy of many secreted therapeutic proteins."
ARIAD Pharmaceuticals is engaged in the discovery and development of novel therapeutics based on signal transduction technology. The company is developing small-molecule drugs to block intracellular signaling pathways that play a critical role in major diseases, including osteoporosis and various immune-related disorders. In addition to ARGENT, ARIAD is developing cellular immunotherapy that utilizes small-molecule drugs to control intracellular signaling pathways in engineered cells.
The Patents (Back to Top)
U.S. Patent No. 6,011,018, issued on January 4, 2000, claims methods for regulated production of secreted proteins and cell surface proteins by administration of a Dimerizer Drug. Among the examples included in the claims are regulated expression of erythropoietin, colony stimulating factors, interferons, various growth factors, insulin, coagulation factors, and chemotactic and anti-chemotactic factors. This patent covers ARIAD's orally active protein therapy products currently are in preclinical development.
U.S. Patent No. 5,994,313, issued on November 30, 1999, claims methods for inducing apoptosis in genetically engineered cells by treatment with a Dimerizer Drug. This patent covers ARIAD's graft-versus-host disease product, which currently is in clinical development.
For more information: Victor M. Rivera, ARIAD Pharmaceuticals Inc., 26 Landsdowne St., Cambridge, MA 02139-4234. Tel: 617-494-0400. Fax: 617-494-8144.
