Are Single-Use Technologies Always The Answer?
By Brian Tham, Peregrine Bio

In recent years, it has become abundantly clear that single-use technologies (SUTs) have won the format wars over reusable stainless steel. It is now very common to see new biopharmaceutical manufacturing plants designed and built without a single piece of reusable product-contact equipment. Proponents argue that SUT offers numerous advantages, such as increased flexibility, reduced cleaning validation requirements, and faster turnaround times. However, beneath the surface, there lies a complex web of considerations that challenge the widely held belief that single-use technologies are unequivocally cost-effective.
The Single-Use Bargain
The decision to go with SUT is essentially a trade of one-time high-cost items (capital equipment and cleaning validation) for higher recurring operational expenses (consumable bags). Other benefits ascribed to SUT include increased flexibility, faster turnaround times, and safety improvements as a result of reduced handling of CIP chemicals. The value proposition for SUT touts lower initial costs and sufficient operational benefits to offset the costs of consumables.
The Buffer Prep Problem
When examining assumptions around the benefits of SUT, the baseline case studies are often built around comparisons with stainless-steel bioreactors. This makes sense — bioreactors are one of the most complicated pieces of bioprocessing equipment, with accordingly complicated cleaning and sterilization validation requirements. Also, any risks associated with bioreactor unit operations inherently carry a high potential impact, due to the high probability of a run failure resulting from a problem encountered while a bioreactor is running. It is very understandable that a manufacturing executive would jump at the opportunity to reduce their initial capital expenditure and eliminate CIP and SIP validation from their risk equations. This often leads to a blanket design decision that sees SUT adopted throughout 100% of a manufacturing facility in all processing areas, for all unit operations.
However, things change in a buffer prep context. Buffer prep equipment rarely presents cleaning validation challenges, and the risk differentials between SUT and stainless steel are often less pronounced. Buffer prep is also an intensively used area, and incremental differences in OPEX for single operational cycles can be amplified depending on process design.
As an example, let’s look at a generic hypothetical chromatography unit operation using the following numbers in Table 1:
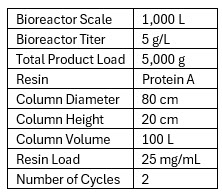
Table 1
These assumptions are intended as much to simplify math as to reflect an actual process, but the intent here is that they are close enough to serve as a useful model.
Cost Considerations
Assuming that our solutions are prepared in a 1,000 L single-use mixer (SUM), the buffer bill for our unit operation is described below in Table 2.
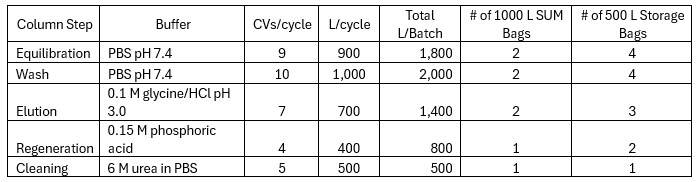
Table 2
Costs of single-use bags can vary, but for the sake of our model, let’s once again make a few assumptions that help to make our math easier:
- Assumed cost of a 1,000 L SUM bag: $5,000
- Assumed cost of a 500 L buffer storage bag: $500
We also can make estimates of the component costs for each of our buffers from readily available pricing information from major suppliers.
This allows us to compare the costs of buffer components for our unit operation with the cost of single-use bags in Table 3.
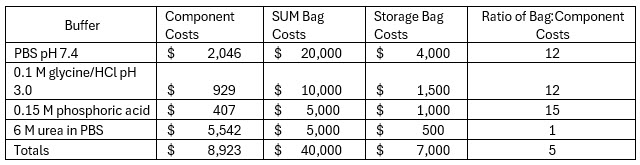
Table 3
As seen here, for most of the buffers used in our example unit operation, the ratio of bag costs to component costs is more than 10x. A cost profile such as this, taken in isolation, would almost surely be challenged. However, due to the reflexive use of a bioreactor as the primary context around decisions related to adopting single-use technologies, scenarios related to buffer prep are often not fully assessed.
If we extend this cost-profile over a one-year period, we can make the following assumptions for a manufacturing facility running for a full year:
- Total manufacturing batches per year: 40
- Total cost of buffer components per year for this unit operation: $356,906
- Total cost of single-use mixer bags per year for this unit operation: $1,600,000
Extending these assumptions across additional unit operations quickly takes the total cost of SUM bags for buffer prep into the mid-seven figures – an amount of money that, if deployed differently, could very likely fund the development and validation of simple CIP/WFI-rinse cycles for fixed buffer prep equipment.
CDMO vs. Internal Manufacturing
SUTs are highly compatible with a CDMO business model. As consumables are treated as pass-through costs, considerations about cost profiles related to SUT are less relevant than considerations related to cleaning validation and product carryover. In an internal manufacturing context, however, a more sophisticated approach to assessing the cost/benefit of SUT can help to optimize the use of SUT across a company’s manufacturing platform.
Cleaning
There is the question of how to estimate the costs of routine cleaning of a fixed stainless-steel vessel. Assuming that a WFI-rinse is sufficient, the single largest driver of cleaning costs will be WFI. Annual operating costs for a WFI system sized at 1,500 L/hr and inclusive of maintenance can be estimated to be on the order of $100,000/year. Looking at our example unit operation, we can estimate the number of CIP cycles required as follows:
- Number of buffer prep events per batch: 8
- Number of manufacturing batches per year: 40
- Total number of WFI-rinse cycles required by example unit operation per year: 320
If our example unit operation is the only operation utilizing the WFI system, our WFI operational costs ($100,000) can be amortized as $312.50 per buffer prep event – less than 10% of our assumed cost for a SUM bag.
It is remarkable how afraid of cleaning validation our industry has become. There is no doubt that providing documentation and ongoing evidence of effective cleaning procedures can be a significant burden on cGMP manufacturing, quality, and regulatory affairs teams. But working within the context of our example, where our cleaning challenge is limited to non-growth-promoting buffers, validating a cleaning cycle for a buffer prep tank would not be expected to be a high-risk activity. Post-validation, modern, at-line TOC analyzers can be implemented for a cost on the order of $40,000/year.
Risk Considerations
The case for SUT often relies on examples of operational risks that are mitigated or eliminated by SUT, such as the risks of cleaning validation failures or contamination. However, years of field experience with SUT and the recent experience of the pandemic have brought to the fore several risks of SUT that have not always been properly accounted for, including:
- Handling Risks: As single-use bags are scaled up, the mechanical stresses that the film layers and joints are subjected to are also scaled up. This results in a certain rate of damage to these bags that is usually not accounted for in cost assumptions.
- Supply Risks: The pandemic underscored the fragility of biopharma supply chains, and in the case of single-use consumables, many single-use items are custom-designed and single-sourced, making them highly vulnerable to disruption.
- Overstated Flexibility: Physical limits on the number of ports and the lead times for establishing new custom bag designs represent significant obstacles to realizing the promised flexibility of a SUT paradigm. Instead, the rise of SUT has tended to drive a convergence of manufacturing process development principles toward the establishment of manufacturing platforms that are applied across portfolios of programs, with many companies adopting an operating philosophy of trying to minimize the number of custom bag designs to be managed in their ERP system.
- Limited Scalability: SUTs become less cost efficient as processes are scaled up or out. Upside scenarios that result in increased demand for products relying heavily on SUTs often result in higher cost of goods where a transition to fixed stainless steel is not made.
- Sustainability Risks: The generation of single-use plastic waste in biopharmaceutical manufacturing processes is an issue that can be thought of as a latent risk for the industry as a whole. Conversations about how best to measure and manage the environmental footprint of cGMP manufacturing operations are ongoing in many quarters but have yet to emerge as a prominent topic. As global trends related to sustainability continue to evolve, it will be important for biopharma manufacturing organizations to stay engaged on this front so as not to fall too far behind the curve.
Conclusion
SUTs have become the industry standard at manufacturing scales below 10,000 L. While the industry has realized tremendous benefits from the adoption of SUT, the current status quo, whereby companies are embracing blanket adoption of SUT for all manufacturing operations, may not be the optimal path forward for all development programs. A thoughtful consideration of possible options for taking a hybrid approach, with reusable stainless-steel equipment implemented for some applications, may enable better operational outcomes for both the clinical and commercial goals of a drug development program.
