Aragen Cell Line Development And Process Development Solutions For Biologics CHO CLD Platforms
Source: Aragen

Aragen offers choice of CLD platforms, efficient Upstream and Downstream process development
CHO CLD platforms
- CHO DG44, CHO GS, CHOZN, SP 2/0
- Our in-house developed CHO DG44 platform can deliver titer >4g/L in 5 months CHO GS: Up to 6g/L- Royalty Free, milestone payment free, difficult-to-express proteins, NBEs, and Animal Health
- Over 100 CLD programs Pre-IND/GMP
- Over 14 in Clinical development and 3+ commercial production
Key Attributes
- RapTr2022 with HIGH TITER and REDUCED TIMELINE
- Royalty-free CHO DG44 best suited for Biosimilar development
- Royalty free, accelerated timeline for DG44 and CHO GS (18 weeks from transfection to RCB)
- Transfection to single cell clone evaluation in 18 weeks with Aragen’s optimized process
- Our CLD platform uses a chemically defined serum free medium and proof of clonality to support IND filing
- Strong Analytical capabilities – Early engagement to establish quality and select right clones to advance
Aragen’s Process Development Services
Aragen offers coefficient upstream and downstream process development
- We use nonproprietary commercially available media and feed for screening studies this allows transfer to any CDMO
- Media and feed screening in Shake Flasks
- Process parameter screening and optimization in AMBR250
- Process consistency and scalability happens in 1L, 5L & 10L Bioreactor

Downstream Process Development
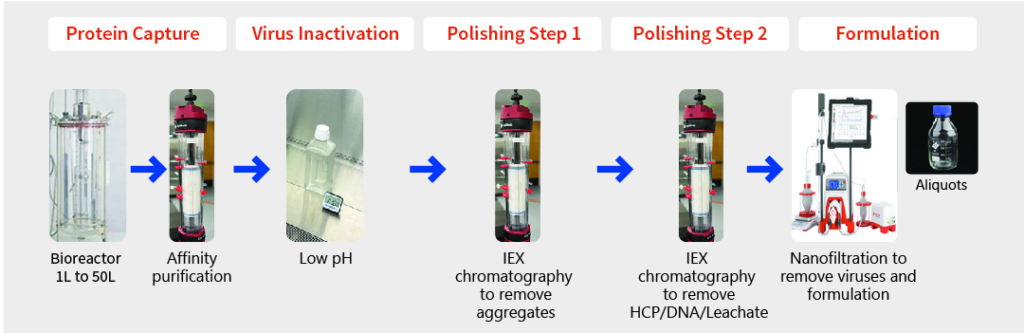
Key Attributes
- Ambr250 platform for Upstream Process development- Synergies to reduce time to IND
- Fully automated AMBR250 24 units, integrated to NOVA Flex-2 & Vi-Cell
- Multifactorial DOE to screen process parameters like- pH, DO, Temp, agitation etc
- Process parameter Optimization and identifying best operative parameters
- History of generating Biosimilar cell line- now in market
