Advanced Analytical Approaches For AAV Empty/Full Capsid Characterization And Quantification
By Joseph Ong, Ph.D., expert, analytical development, Novartis
Adeno-associated viruses (AAVs) are an emerging vector for gene therapy. AAVs package a therapeutic DNA payload within a viral capsid. This DNA payload is limited to about 5 kb of DNA and can be used for gene editing, gene silencing, or gene replacement. Once the AAV reaches the target cell, the AAV is internalized and trafficked to the nucleus, where the DNA payload is delivered and the therapeutic product is expressed.
There are many advantages to using AAVs for gene therapy. AAVs are generally considered safe, can be delivered directly into a patient, and can target different tissues and cross the blood brain barrier. The capsids can also be engineered to increase tissue specificity, potency, and safety. As a result, many AAV-based gene therapies are being developed, and some have been approved by global regulatory authorities.
The empty/full capsid ratio is an important CMC consideration. A well-controlled process will produce ratios of empty, partial, and full capsids that are comparable between lots. Appropriate methods for quantifying the empty/full ratio of AAVs are thus important analytical tools. However, there are many techniques for doing so, and choosing which one is most appropriate can be a challenge. Here, 10 different techniques will be presented and evaluated for their analytical strength, ease of use, and their ability to be implemented in a GMP environment.
The AAV Empty/Full Capsid Ratio Is An Important Quality Attribute
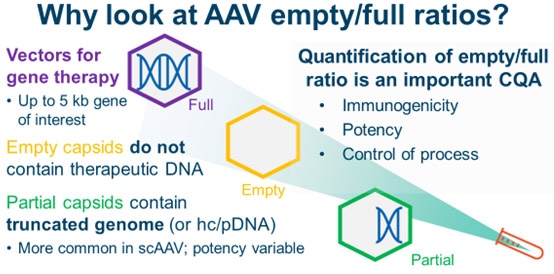
AAVs that contain the desired DNA product are considered full capsids. Besides full AAVs, however, other capsid types can be present in the final formulation. Empty capsids do not contain DNA of interest. They might be truly empty, or they might contain small pieces of DNA, like AAV ITRs or fragments of host cell DNA or plasmid DNA. Partial capsids may contain pieces of the full AAV genome. Partial capsids are more prevalent in programs with self-complementary AAV genomes than those with single-stranded AAV genomes.
Full AAVs are the most potent: they contain the desired DNA product. Empty capsids, which have no therapeutically useful DNA, make no contribution to the potency of the product. Instead, they may contribute to immunogenicity by delivering immunogenic fragments of DNA and heightening the patient’s immune system response against AAV capsids. Partial capsids have varying potency depending on the DNA that is packaged. If the packaged genome happens to code for a functional protein, a partial capsid may positively contribute to potency. As a result, in addition to improving overall capsid yield, process development groups will try to optimize the percentage of full capsids.
Methods For AAV Empty/Full Determination
Characterizing and quantifying the AAV empty/full ratio fall along four main methods:
- Separate measurements of the DNA titer and capsid titer
- Separation based on charge differences between empty and full capsids
- Separation based on mass and size
- Imaging techniques
Measurements of DNA and capsid
ddPCR and ELISA
The principle behind using ddPCR and ELISA to measure the empty/full capsid ratio is simple: ddPCR measures the DNA concentration (number of vector genomes/mL), ELISA measures the protein concentration (number of capsids/mL), and the ratio of DNA to capsid is calculated (number of vector genomes/number of capsids) to give the number of full and empty capsids.
While this approach is easy to perform and does not require any specialized equipment, it has its disadvantages. ddPCR cannot quantify partial capsids. Depending on the primer design and the sequence of the partial genome, primers will either recognize the partial genome, resulting in an overestimation of the number of full capsids, or the primers will not recognize the partial genome and overestimate the number of empty capsids. ELISAs are dependent on the accuracy and availability of the antibody. Antibodies can recognize capsid fragments and may be subjected to lot-to-lot variation. A poor calibration curve may affect the calculated capsid titer. Finally, because using ddPCR and ELISA as a measure of the empty/full ratio involves combining the results of two separate techniques, the reported result involves the compounding of two sources of error, which may lead to high assay variability.
UV-Vis
UV-Vis spectroscopy takes the molar absorptivity coefficients for the DNA genome and the protein capsid and calculates how much DNA and protein are present within the sample. Software can be used to calculate the number of vector genomes and the number of capsids, and, like ddPCR and ELISA, the ratio of empty and full capsids is measured. Because partial capsids are not separated from the empty and full capsids, the signals from partial capsids are assigned to empty and full capsids.
Because there is no separation of capsids, both DNA and protein impurities will affect the UV-Vis reading. In our experience, UV-Vis is most accurate with very pure samples. In our experience, however, even with the purest samples, UV-Vis often significantly overestimates the number of empty capsids. UV-Vis may be a useful method for groups trying to get a fast (but not necessarily accurate) titer value.
Size exclusion chromatography – multi angle light scattering (SEC-MALS)
SEC-MALS couples size exclusion chromatography (which separates the monomeric AAVs from dimers, aggregates, and other impurities) with an analytical suite of detectors such as light scattering, UV detection, and refractive index detectors. Together, these detectors can report the size of the AAVs in solution, capsid titer, and genomic titer. However, because the empty, partial, and full capsids elute together as one monomer peak, SEC-MALS will partition signals from partial capsids between empty and full capsids, similar to UV-Vis.
In our experience, SEC-MALS is a strong analytical technique with reasonable linearity and is reproducible. In terms of its weaknesses, we have seen that SEC-MALS will occasionally overestimate the number of empty capsids. Its software may also be a bit tricky to use. Because SEC-MALS cannot distinguish partial capsids, it may be more suitable for AAV projects with few partial capsids.
Charge-based separations
Full capsids have a lower pI (are more negatively charged, are more acidic) than empty capsids. This difference allows for separations based on pI. In our experience, partial and full capsids have similar pIs, making separation of these two species challenging. Charge-based separations are complicated by post-translational modifications, which may affect the surface charge. These separations are also serotype-dependent, meaning one method may not easily translate to another program.
Anion exchange chromatography (AEX)
In AEX, the more negatively charged full and partial capsids are retained longer on the column than the earlier eluting empty capsids. In a good AEX method, empty and full capsids are separated from each other with baseline resolution. As a column-based method, AEX is easy to perform, robust, and accurate.
However, establishing an AEX method can be challenging, as the choice of column, salt type, concentration, and buffer pH can all affect the quality of the separation. Moreover, some serotypes are more challenging to analyze via AEX.
Capillary isoelectric focusing (cIEF)
cIEF separates empty and full capsids along a pH gradient according to their pI. With a good cIEF method, intact empty and full capsids will separate, allowing for quantification. cIEF requires a high-purity sample, as impurity peaks may not easily be distinguishable from protein signals. While cIEF may give high-resolution information on the charge heterogeneity within the sample, a complete separation of empty and full capsids may be challenging. Post-translational modifications, which can cause the peaks to merge into each other, can further complicate this analysis.
Mass and size separations
Sedimentation velocity – analytical ultracentrifugation (SV-AUC)
SV-AUC is a form of centrifugation where AAVs species are separated by their Svedberg coefficient, which is a measure of a particle’s mass, size, and its shape. Broadly, empty capsids have a lower Svedberg coefficient than full capsids. In addition to providing high-resolution separation of empty and full capsids, SV-AUC can also separate partial capsids.
However, SV-AUC is challenging to implement in a GMP setting. It requires a large amount of manual labor, and data analysis is time-consuming. Nonetheless, because of its ability to accurately separate and quantify empty, partial, and full capsids, SV-AUC is the gold standard for many AAV programs.
Charge detection mass spectrometry (CDMS)
CDMS is an emerging form of mass spectrometry where both the mass-to-charge ratio (m/z) and the charge (z) of the intact capsid are simultaneously determined. Multiplying the two values together allows for the determination of the intact capsid mass m. For a 5.1 MDa full capsid, typical m/z values are 30,000 and +170 for charge (30,000 m/z * 170 z = 5,100,000 Da). In our experience, CDMS and AUC results match very closely, meaning that CDMS may be an appropriate substitute for AUC in analytical labs.
Atomic force microscopy (AFM)
AFM uses a cantilever to probe the physiochemical characteristics of AAVs deposited onto an imaging surface. Empty capsids are “squishier” and appear smaller than full capsids.1,2 Differences in hydrophobicity and surface charge can also be observed.3 However, as characteristics of empty and full capsids may overlap or the differences may be subtle, AFM may be better suited as a characterization method instead of an empty/full determination method.
Imaging techniques
Cryogenic transmission electron microscopy (cryoTEM)
CryoTEM is an imaging technique that measures the electron density within an AAV capsid. Full capsids have high electron density (high contrast) within their interiors due to the presence of DNA while empty capsids will have little contrast. CryoTEM can also try to quantify capsids with medium contrast as partial capsids, but a good algorithm is necessary for deciding what is a low (empty), medium (partial), or high (full) contrast capsid. We have also observed that cryoTEM may overestimate the number of empty capsids within our samples.
Mass photometry
Mass photometry is an imaging technique that looks at the amount of light scattered (or increase in contrast) when an AAV lands on a microscope slide. A full AAV will show up as a darker spot (have greater contrast) on a microscope slide than an empty AAV. By measuring the number of AAVs landing on a microscope slide and the contrast or intensity of the landing event, the empty/full ratio can be determined.
One advantage of mass photometry is that it is a fast technique (about five minutes per sample) and easy to perform. As a single-molecule technique, it provides highly detailed information on the heterogeneity of the sample. However, while mass photometry can distinguish partial capsids, the resolution is not very good, meaning that the distributions of partial capsids and full capsids may bleed into each other. Our preliminary results with the instrument suggest that mass photometry misassigns partial capsids as full capsids.
Choosing An Empty/Full Method
Choosing an empty/full quantification method is highly context-dependent. No single technique is accurate, fast, easy to use, and easy to implement in a GMP environment. If partial capsids are a significant portion of the drug substance, then SV-AUC, CDMS, and mass photometry are the most reasonable methods. While CDMS may be the easiest to perform, SV-AUC has the advantage of being possible to implement in GMP environments. Because mass photometry is fast and easy to perform, it may be useful for process development teams to screen through various processes.
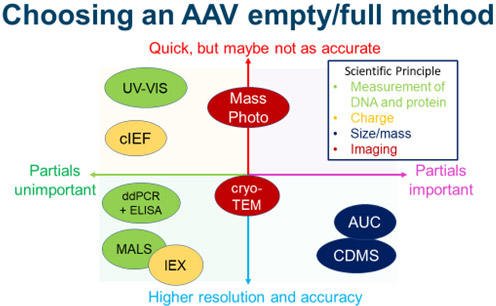
If partial capsids are not a concern, then column-based methods should be strong candidates. If the serotype is amenable to ion exchange chromatography, anion exchange chromatography is an accurate and GMP-friendly technique. SEC-MALS is also a reasonable choice, though care must be taken to make sure the method is accurate.
Cryo-TEM stands out as a middling technique because it has a questionable ability to distinguish partial capsids and mediocre accuracy. UV-Vis may be useful for a process team interested in quickly estimating the empty/full capsid ratio. Unless the method can completely separate empty and full particles, cIEF and AFM may be better as characterization techniques than methods of absolute quantification. Finally, ddPCR and ELISA can be used if necessary, but these approaches must be thoroughly validated to ensure the result is accurate.
Acknowledgements
The author thanks his colleagues Yueju (Julia) Wang, Edward Towers, Judit Bartalis, Marianne Goodwin, and Mouzhong Xu for providing samples, expertise, and data necessary for technique comparison and writing this manuscript.
References:
- Chen, Heng. "Atomic Force Microscopy of Recombinant Adeno‐associated Virus‐2 Prepared by Centrifugation." Scanning: The Journal of Scanning Microscopies 29.5 (2007): 238-242.
- Nam, Yu Ri, et al. "Distinguishing between DNA-Loaded Full and Empty Capsids of Adeno-Associated Virus with Atomic Force Microscopy Imaging." Langmuir (2023).
- Heldt, Caryn L., et al. "Empty and Full AAV Capsid Charge and Hydrophobicity Differences Measured with Single-Particle AFM." Langmuir (2023).
About the Author:
 Joseph Ong, Ph.D., is currently an expert in analytical development, chemistry in the Cell and Gene Therapy division of Novartis Pharmaceuticals. Before joining Novartis, he completed his Ph.D. in June 2022 at UCLA. He currently focuses on developing analytical tools for the analysis of the AAV empty/full ratio, protein and DNA impurities, and characterization of AAVs.
Joseph Ong, Ph.D., is currently an expert in analytical development, chemistry in the Cell and Gene Therapy division of Novartis Pharmaceuticals. Before joining Novartis, he completed his Ph.D. in June 2022 at UCLA. He currently focuses on developing analytical tools for the analysis of the AAV empty/full ratio, protein and DNA impurities, and characterization of AAVs.
