A SMART Approach To CAPA Effectiveness Checks
By Mark Durivage, Quality Systems Compliance LLC
Corrective and preventive action (CAPA) issues continue to be one of the top Form 483 observational findings by the FDA. Many times, CAPAs fail due to the structure and flow of the process and not necessarily the efforts of those managing the CAPAs. Frequently, organizations do not fully document the CAPA phases and confuse verification of implementation and verification of effectiveness activities. (For an introduction to the CAPA phases, read my previous article “The 10 Phases Of An Effective CAPA.”) This article will look at using the SMART (specific, measurable, achievable, relevant, and time-bound) methodology for use in developing a CAPA verification of effectiveness plan.
FDA. Many times, CAPAs fail due to the structure and flow of the process and not necessarily the efforts of those managing the CAPAs. Frequently, organizations do not fully document the CAPA phases and confuse verification of implementation and verification of effectiveness activities. (For an introduction to the CAPA phases, read my previous article “The 10 Phases Of An Effective CAPA.”) This article will look at using the SMART (specific, measurable, achievable, relevant, and time-bound) methodology for use in developing a CAPA verification of effectiveness plan.
CAPA Requirements
Several international regulations and standards provide guidance on conducting CAPAs:
21 CFR 820 Quality System Regulation
Sec. 820.100 Corrective and preventive action:
(a) Each manufacturer shall establish and maintain procedures for implementing corrective and preventive action. The procedures shall include requirements for:
(1) Analyzing processes, work operations, concessions, quality audit reports, quality records, service records, complaints, returned product, and other sources of quality data to identify existing and potential causes of nonconforming product, or other quality problems. Appropriate statistical methodology shall be employed where necessary to detect recurring quality problems;
(2) Investigating the cause of nonconformities relating to product, processes, and the quality system;
(3) Identifying the action(s) needed to correct and prevent recurrence of nonconforming product and other quality problems;
(4) Verifying or validating the corrective and preventive action to ensure that such action is effective and does not adversely affect the finished device;
(5) Implementing and recording changes in methods and procedures needed to correct and prevent identified quality problems;
(6) Ensuring that information related to quality problems or nonconforming product is disseminated to those directly responsible for assuring the quality of such product or the prevention of such problems; and
(7) Submitting relevant information on identified quality problems, as well as corrective and preventive actions, for management review.
(b) All activities required under this section, and their results, shall be documented.
ISO 13485:2016 Medical devices — Quality management systems — Requirements for regulatory purposes
8.5.2 Corrective action
The organization shall take action to eliminate the cause of nonconformities in order to prevent recurrence. Any necessary corrective actions shall be taken without undue delay. Corrective actions shall be proportionate to the effects of the nonconformities encountered.
The organization shall document a procedure to define requirements for:
a) reviewing nonconformities (including complaints);
b) determining the causes of nonconformities;
c) evaluating the need for action to ensure that nonconformities do not recur;
d) planning and documenting action needed and implementing such action, including, as appropriate, updating documentation;
e) verifying that the corrective action does not adversely affect the ability to meet applicable regulatory requirements or the safety and performance of the medical device;
f) reviewing the effectiveness of corrective action taken.
Records of the results of any investigation and of action taken shall be maintained.
8.5.3 Preventive action
The organization shall determine action to eliminate the causes of potential nonconformities in order to prevent their occurrence. Preventive actions shall be proportionate to the effects of the potential problems.
The organization shall document a procedure to describe requirements for:
a) determining potential nonconformities and their causes;
b) evaluating the need for action to prevent occurrence of nonconformities;
c) planning and documenting action needed and implementing such action, including, as appropriate, updating documentation;
d) verifying that the action does not adversely affect the ability to meet applicable regulatory requirements or the safety and performance of the medical device;
e) reviewing the effectiveness of the preventive action taken, as appropriate. Records of the results of any investigations and of action taken shall be maintained.
The Joint IPEC - PQG Good Manufacturing Practices Guide for Pharmaceutical Excipients
8.5.2 Corrective Action
The excipient manufacturer should establish, document and maintain procedures for:
- determining the root causes of nonconformities,
- ensuring that corrective actions are implemented and effective,
- implementing and recording changes in procedures resulting from corrective action.
8.5.3 Preventive Action
The excipient manufacturer should establish, document and maintain procedures for:
- initiating preventive actions to deal with problems at a level corresponding to the risks,
- implementing and recording changes in procedures resulting from preventive action.
ICH Harmonised Tripartite Guideline Pharmaceutical Quality System Q10
3.2.2 Corrective Action and Preventive Action (CAPA) System
The pharmaceutical company should have a system for implementing corrective actions and preventive actions resulting from the investigation of complaints, product rejections, non-conformances, recalls, deviations, audits, regulatory inspections and findings, and trends from process performance and product quality monitoring. A structured approach to the investigation process should be used with the objective of determining the root cause. The level of effort, formality, and documentation of the investigation should be commensurate with the level of risk, in line with ICH Q9. CAPA methodology should result in product and process improvements and enhanced product and process understanding.
Applying The SMART Methodology To CAPAs
An effective complaint CAPA process consists of 10 distinct phases, as shown in the figure below. Phase 8, verification of effectiveness plan (VOEP), is rarely used, yet it can cause a CAPA to fail during the verification of effectiveness check. The VOEP is used to establish and document predetermined criteria to verify that corrective and/or preventive actions were indeed effective.
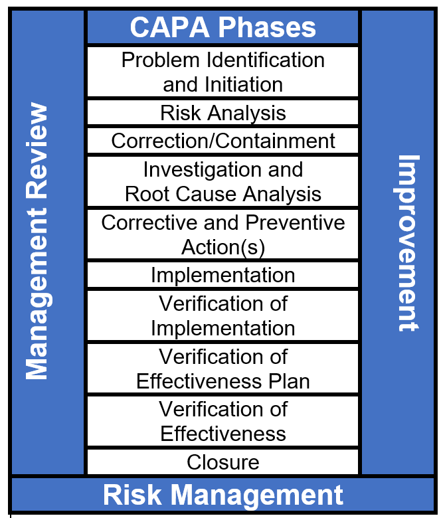
George T. Doran is credited with first using the term SMART in the November 1981 issue of Management Review. Peter Drucker's management by objectives concept utilized SMART goals. Today, the SMART methodology is generally associated with personal goals and performance objectives related to raises and annual bonuses, but the methodology can be readily be applied to CAPA VOEP activities.
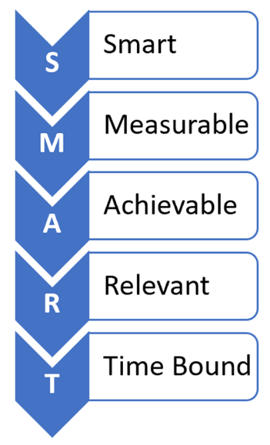
When developing and evaluating a SMART VOEP, the following questions should be considered:
- Specific — Is the VOEP unambiguous, clear, and focused?
- Measurable — Is quantifiable data being used to assess the VOEP?
- Achievable — Is the VOEP feasible or practical?
- Relevant — Is the VOEP appropriate for the level of risk?
- Time-Bound — Does the VOEP have a realistic deadline?
Additionally, reasonable and realistic are acceptable substitutes for the R or relevant question.
Examples of a VOEP’s statements include:
- The problem has not recurred in the last three production lots.
- The problem has not recurred in the last three months.
- The first pass yield will increase from 90 to >99 percent during Q2 2020.
- The scrap rate will decrease by 5 percent (say, from 15 percent to 10 percent) over three months.
The verification of effectiveness phase is the process of reviewing the criteria and documenting the evidence that was predetermined in the VOEP prior to closure. Just as in process validation activities, if the predetermined acceptance criteria are not met, the CAPA fails. When a verification of effectiveness fails, close the CAPA as ineffective and open a new CAPA. Ensure the new CAPA references the old CAPA.
VOEP Example
A short time ago, a mature stable package sealing process running on Heat Sealer 20 began occasionally producing parts that were not meeting the approved pull test specification that was necessary to maintain package integrity. A typical production run is approximately 1,000 packages, which is easily completed during a normal workday for the facility.
The production manager opened CAPA 2020-005 as a result of the nonconforming package seals being produced. A CAPA team was formed, an impact assessment was conducted, and containment actions were performed due to the risk involved with the defective package seals. Production records, nonconformances, calibrations, maintenance records, training records, and validation documentation for the past year were reviewed, and the heat sealer machine operators were interviewed. A root cause investigation was conducted, a root cause determined, and the corrective actions were implemented.
During the review of the production records, nonconformances, calibrations, maintenance records, training records, validation documentation, and operator interviews, the CAPA team’s investigation concluded machine settings on Heat Sealer 20 were sometimes set outside the validated parameters by new, inexperienced heat sealer machine operators. The CAPA team concluded this was the most likely root cause of the nonconforming package seals.
The CAPA team decided to address the root cause by changing the programmable logic controller user access permissions, which effectively restricted the operators’ ability to select parameters that were outside the validated settings on the heat sealer. The heat seal machine operators will be retrained to reinforce the importance of using the validated machine parameters and that these validated settings are crucial to prevent package seal failures. Additionally, the CAPA team initiated a change order to require the actual heat sealer machine settings to be recorded in the production record at the beginning and end of the production record to verify the settings were within the validated ranges.
The production manager, in conjunction with the CAPA team, used the SMART method to develop the VOEP. The CAPA team decided on the following VOEP: The packages sealed on Heat Sealer 20 will be monitored for conformance to specifications (seal integrity failures) over three consecutive days of production (three lots) using a tightened level of inspection per the sampling procedure. The CAPA will be deemed effective if all of the packages produced for the next three consecutive days of production (three lots), meet the pull test specification, the operators are unable to select parameters outside of the validated range, and the proper validated settings are recorded in the batch record.
The CAPA team performed a verification of implementation assessment to verify the heat sealer operators were retrained, the programmable logic controller user access permissions restrict the operators’ ability to select parameters that are outside the validated settings on the heat sealer, and the production record documentation was updated to document the settings are within the validated ranges. The CAPA team concluded the verification of implementation tasks was completed as planned.
For the next step the CAPA team evaluated the VOEP to determine whether it’s SMART.
Specific — Is the VOEP unambiguous, clear, and focused? ☑ Yes ☐ No
- The packages produced for the next three consecutive days of production (three lots) on Heat Sealer 20 must meet the pull test specification.
- The operators are unable to select parameters outside of the validated range.
- The proper validated settings are recorded in the batch record.
Measurable — Is quantifiable data being used to assess the VOEP? ☑ Yes ☐ No
- The packages produced for the next three consecutive days of production (three lots) on Heat Sealer 20 must meet the pull test specification.
- The operators are unable to select parameters outside of the validated range.
- The proper validated settings are recorded in the batch record.
Achievable — Is the VOE feasible or practical? ☑ Yes ☐ No
- The packages produced for the next three consecutive days of production (three lots) on Heat Sealer 20 must meet the pull test specification.
Relevant — Is the VOE appropriate for the level of risk? ☑ Yes ☐ No
- The packages sealed on Heat Sealer 20 will be monitored for conformance to specifications (seal integrity failures) over three consecutive days of production (three lots) using a tightened level of inspection per the sampling procedure.
Time-Bound — Does the VOE have a deadline? ☑Yes ☐ No
- The packages produced for the next three consecutive days of production (three lots)
The CAPA team performed the VOE following results:
☑ Yes ☐ No Heat Sealer 20 ran for three consecutive days of production (three lots) using the validated settings.
☑ Yes ☐ No All packages meet the pull test specification.
☑ Yes ☐ No The validated heat sealer settings for the beginning and end of each production run were properly recorded in the batch record.
The CAPA team decided the CAPA was complete and effective. The team also shared its success with other departments so similar actions could be implemented.
The example demonstrates the appropriate use of the SMART method to plan and verify CAPA effectiveness, ultimately resulting in CAPA closure. Adapting the SMART method can help improve the effectiveness of your CAPA program and demonstrate compliance with regulations, standards, and expectations.
Conclusion
Not only are CAPAs required by regulations, standards, and guidances, CAPAs, if performed correctly, can help an organization improve its competitive position by reducing waste and improving processes. Using the SMART methodology is an effective way to develop sound verification of effectiveness plan, leading to a compliant CAPA system.
About the Author
 Mark Allen Durivage has worked as a practitioner, educator, consultant, and author. He is managing principal consultant at Quality Systems Compliance LLC, an ASQ Fellow, and an SRE Fellow. He earned a BAS in computer aided machining from Siena Heights University and an MS in quality management from Eastern Michigan University. He holds several certifications, including CRE, CQE, CQA, CSQP, CSSBB, RAC (Global), and CTBS. He has written several books available through ASQ Quality Press, published articles in Quality Progress, and is a frequent contributor to Life Science Connect. Durivage resides in Lambertville, Michigan. Please feel free to email him at mark.durivage@qscompliance.com or connect with him on LinkedIn.
Mark Allen Durivage has worked as a practitioner, educator, consultant, and author. He is managing principal consultant at Quality Systems Compliance LLC, an ASQ Fellow, and an SRE Fellow. He earned a BAS in computer aided machining from Siena Heights University and an MS in quality management from Eastern Michigan University. He holds several certifications, including CRE, CQE, CQA, CSQP, CSSBB, RAC (Global), and CTBS. He has written several books available through ASQ Quality Press, published articles in Quality Progress, and is a frequent contributor to Life Science Connect. Durivage resides in Lambertville, Michigan. Please feel free to email him at mark.durivage@qscompliance.com or connect with him on LinkedIn.
