A Robust Approach to Differential Expression Analysis for Both Prokaryotic and Eukaryotic Systems
By Paul Diehl, Director of Sales and Marketing, Display Systems Biotech
Contents
Advantages of RFDD-PCR
Limitations of Using Anchored Poly-dT Primers
Expression Profiling in Prokaryotic Systems
RFDD-PCR Anaylsis of Antibiotic Resistance in Listeria monocytogenes
References
Advantages of RFDD-PCR (Back to Top)
Restriction fragment differential display (RFDD-PCR)—commercialized by Display Systems Biotech in the displayPROFILE System—offers a novel approach to identify differentially expressed genes in both prokaryotic and eukaryotic organisms. In addition to overcoming many disadvantages of other gel-based techniques that analyze differential gene expression, RFDD-PCR's unique approach always produces the same cDNA fragments at the same location in a profile from a particular gene. That is, the position on the expression profile gels of the fragments produced from a specific gene can be predicted for any known sequence before the analysis is performed. This feature of RFDD-PCR results in consistent and reliable results. Further, this attribute allows a researcher to search relevant sequence databases and identify genes that produce bands of the same size and location to interesting differentially expressed bands present on an expression profile. This dimension of analysis, which provides a researcher with a lot of information simply by analyzing the expression profile, is only possible with the RFDD-PCR technique.
In addition to the predictive nature of the approach, RFDD-PCR is also more reproducible and consistent than traditional differential display (1). In using low-stringency PCR conditions with arbitrary primers of 10–12 nucleotides in length and anchored poly-dT primers, traditional differential display is very sensitive to cycling conditions, so much so that the results often vary significantly as a result of minor temperature profile variation from tube to tube. Instead of arbitrary primers, RFDD-PCR uses primers that bind specifically to adaptor sequences and stringent PCR with high-temperature annealing. These conditions give highly resolved discrete bands, which can be reliably reproduced from experiment to experiment.
Limitations of Using Anchored Poly-dT Primers (Back to Top)
Using anchored poly-dT primers in traditional differential display primarily limits amplification to the 3'-untranslated regions, rather than the coding regions of genes, which are the most interesting. With RFDD-PCR, restriction enzyme-digested cDNA is ligated with adaptors, which allows amplified cDNA fragments to originate from any portion of the sequence. Thus, preferential amplification of the 3'-untranslated regions does not occur with RFDD-PCR.
In addition to primarily amplifying 3'-untranslated regions, the use of anchored poly-dT primers in traditional differential display limits it to eukaryotic systems since prokaryotes do not have polyadenylated mRNA. To date, only a few attempts has been made to adapt traditional differential display for prokaryotic gene profiling (2–4). Generally, these adaptations make use of RNA arbitrarily primed (RAP) PCR. With this technique, either single arbitrary primers or random hexanucleotide mixtures are chosen for the RT reaction, and the subsequent PCR reactions contain one or two arbitrary primers. Although this approach overcomes the limitation of using anchored poly-dT primers, using two arbitrary primers for amplification introduces a strong bias for the amplification for particular groups of genes. In addition, these approaches still suffer from the problems introduced by using promiscuous PCR conditions with arbitrary primers—namely, a high number of false positives and problems with reproducibility.
Expression Profiling in Prokaryotic Systems (Back to Top)
As a result of its unique approach, the RFDD-PCR technology (see Fig. 1) can be directly applied to prokaryotic, as well as eukaryotic systems. For prokaryotic systems, only a single modification is necessary in the synthesis of the single-stranded cDNA. Instead of using poly-dT for reverse transcription, a randomized 6 or 8 base oligonucleotide primes the reaction. Following this synthesis, the rest of the procedure is the same for prokaryotic systems as with eukaryotic systems.

Fig 1. Restriction-Fragment Differential Display-PCR Procedure (RFDD-PCR).
Synthesized double-stranded cDNA is digested with the TaqI restriction enzyme, which has a 4-base recognition sequence. Following digestion, two specially designed DNA adaptors are ligated to the cDNA fragments. One adaptor lacks a portion of the complementary strand and has a modification on the 3'end, an "extension protection group" (EPG), which prevents the polymerase from filling-in this missing sequence. Using this adaptor is essential to the RFDD-PCR procedure. One of the primers used in the PCR reactions (the 0-extension primer) binds to the sequence missing from this EPG-adaptor. Thus, priming with the 0-extention primer does not occur until the binding site for this primer is synthesized when the complementary strand is synthesized during the first round of PCR. This approach allows the primer that binds to the other standard adaptor to determine which cDNA fragments are amplified in the reaction.
The other primer used in the PCR reaction binds to the regular adaptor and extends 3 bases past the adaptor junction into the cDNA fragment. Since the 0-extension primer cannot bind for the first round of PCR, these 3 bases on this primer determine which cDNAs are amplified in a specific reaction. Thus, each PCR reaction only amplifies a specific subset of cDNA fragments that have a specific three base sequence upstream of the adaptor ligation site. This subset of amplified cDNAs is referred to as an expression window. To amplify all variations—all expression windows, which provides a complete cross section of all the expressed sequences—only 64 (i.e., 43) different primers are necessary. For prokaryotes, which typically express only a few thousand genes, it is sufficient to divide the gene fragment pool into 32 expression windows instead of 64. Thus, the 3-base extension primers can be degenerate in the third position (e.g., A A A/G or C T A/G) and still selectively amplify a small enough subset of the expressed cDNA to provide interpretable results.
As mentioned previously, the advantage of this approach is it makes it possible to predict the size and position—specifically, in which of the 32 prokaryotic expression windows—the TaqI fragments for particular reported genes will appear. Conversely, a sequence database can be searched to identify particular genes that would produce bands matching those of interest in a profile. An example of such an analysis for E. coli is shown in Fig. 2. It should be noted that, for relatively simple genomes like those in E. coli, only one or a few gene candidates will match these search criteria. Thus, it is possible to determine the identity of many of the gene fragments of interest in an RFDD-PCR profile without excising and sequencing the particular bands.
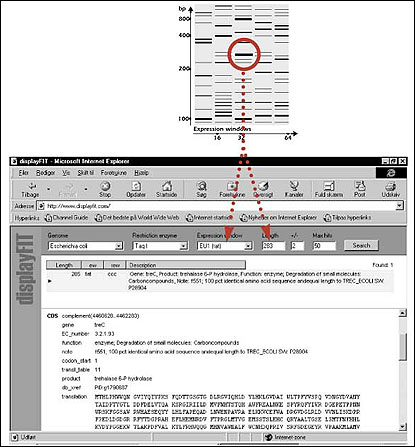
Fig. 2. Use of the displayFIT Program to identify differentially regulated genes based on the position of gene fragments found in an RFDD-PCR profile.
RFDD-PCR Anaylsis of Antibiotic Resistance in Listeria monocytogenes (Back to Top)
In an application of RFDD-PCR expression profiling in a prokaryotic system, we recently analyzed spontaneously developed resistance towards antibiotics in Listeria monocytogenes—an important target organism for food preservatives. Specifically, we looked at resistance to the bacteriocins pediocin and nisin. With RFDD-PCR, we identified numerous genes that were consistently overexpressed in several independent pediocin-resistant and nisin-resistant mutants (see Fig. 3).
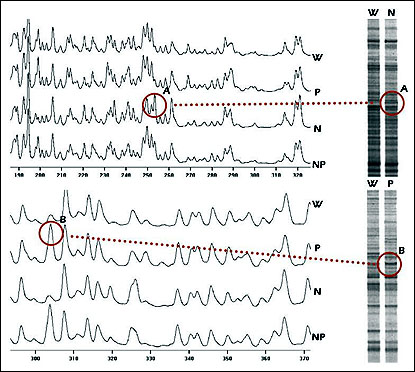
Fig. 3. Fluorescence and isotopic RFDD-PCR analysis of Listeria monocytogenes resistance to bacteriocins. Cultures of Listeria monocytogenes were treated with the bacteriocins nisin and pediocin either alone or in combination. RNA from resistant cultures was isolated analyzed using RFDD-PCR. Results from both the fluorescence and isotopic analysis are shown.
After analyzing, excising, and sequencing the bands that were differentially expressed in the bacteriocin-resistant mutants, two novel bacteriocin resistance genes were found—one involved in resistance towards pediocin and another involved in the resistance towards nisin. Their general involvement in bacteriocin resistance was confirmed by Northern blotting (see Fig. 4). These results indicate the general usefulness of RFDD-PCR for analyzing correlated changes in bacterial gene expression.

Fig. 4. Northern blots of novel genes involved in Listeria monocytogenes resistance towards nisin and pediocin.
- Liang, P., and Pardee, A. (1992) Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction <> 257, 967-971.
- Kwaik, Y.A., and Pederson, L.L. (1994) Cloning and molecular characterization of a Legionella pneumophila gene induced by intracellular infection and by various in vitro stress conditions. Mol. Microbiol., 21, 543-556.
- Wong, K.K., and McClelland, M. (1994) Stress-inducible gene of Salmonella typhimurium identified by arbitrarily primed PCR of RNA. Proc. Natl. Acad. Sci. USA, 91, 639-643.
- Fislage, R., Berceanu, M., Humboldt, Y., Wendt, M., and Oberender., H. (1997) Primer design for a prokaryotic differential display RT-PCR. Nucl. Acids Res. 25, 1830-1835.
To send feedback on this article in BioResearch Online's discussion forum, click here.
For more information, contact: Paul Diehl, Director, Marketing and Sales, Display Systems Biotech. Tel: 800-697-1111 or 760-599-0598. Fax: 760-599-9930. Email: pd@displaysystems.com.
