A Practical Proposal For Standardizing Traceability Of Cell And Gene Therapies
By BioPhorum
For personalized therapies, it is imperative that patients are treated with the product meant specifically for them, whether it is:
- made from their own cells (an autologous therapy),
- individualized based on data derived from their cells (e.g., cancer vaccines that are personalized based on genetic sequencing data), or
- simply dosed according to the characteristics of the patient (e.g., some weight-based gene therapies or precision allogeneic cell therapies).
Mistakes in products or on labels that lead to the wrong therapy being administered to the patient are likely to have fatal consequences. Traceability is therefore a critical quality attribute for patient safety for cell and gene therapies (CGTs). It goes beyond the batch tracking and serialization of other pharmaceutical products and must also include a full trace back to the starting material and the patient.
This article presents a practical vision for standardizing the traceability of CGTs across the value chain. It is a joint statement of traceability services, which will help create a common language and shape a common baseline for CGT supply processes, enabling partnerships between organizations to become swifter and more flexible.
Chain Of Custody And Chain Of Identity
Chain of custody (COC) is the permanent capture of data related to who handled the collection and/or product, what actions were performed, and the location/date/time of the actions from the start of the collection of starting material through to product administration. Chain of identity (COI) is the permanent, unequivocal, and transparent association that connects a patient’s tissue or cells (starting material) and the resulting drug product with unique identifiers for the patient, for the entire process from order through manufacturing to treatment. Although COC is a familiar concept in traditional pharmaceutical manufacturing, the combination of COC/COI traceability is unique to CGTs.
To ensure COC, there must be a complete, gapless audit trail for every step of the process, documenting who was responsible for the materials and actions at every step. The COC must span all the organizations in the product journey, paying particular attention to the handoffs between them and the individual steps.
Conventionally, the final products are labeled with the patient’s name and date of birth as an easy cross-check for healthcare professionals. This is preferred by the hospitals and supports a zero-error value chain. But the General Data Protection Regulation and the Health Insurance Portability and Accountability Act require the careful management of such patient-identifiable data. Solutions must resolve the tension created by those requirements, satisfying both the labeling and the data privacy aspects.
Tracing cells for a personalized cell or gene therapy must be even better than Mumbai’s famous lunchbox delivery system (see Figure 1) because patients’ lives depend on it.
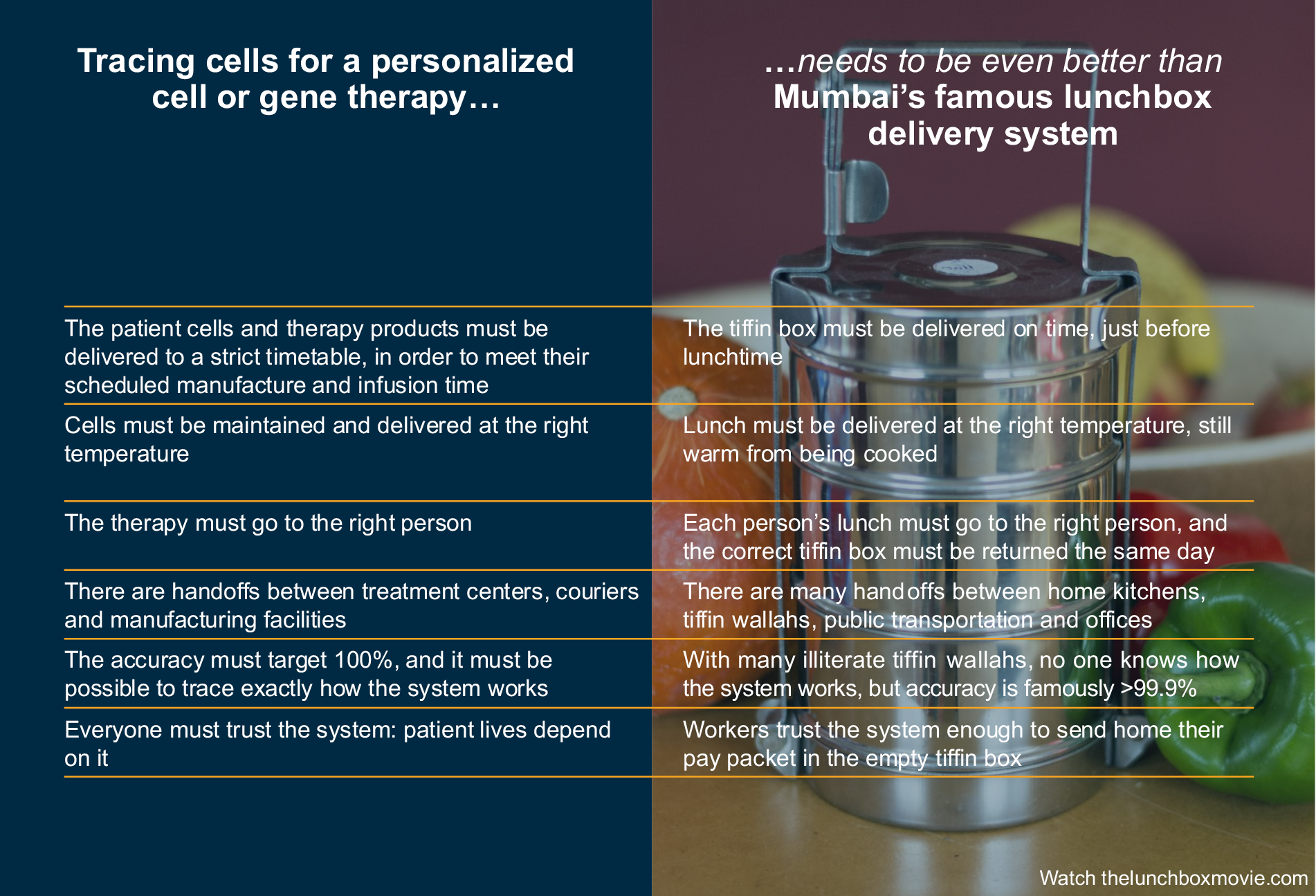
Figure 1: Comparing tracing cells for a personalized CGT to Mumbai’s lunchbox delivery system. Click on image to enlarge.
Personas
Traceability is one of the more complex aspects of the CGT ecosystem. It runs through all parts of the supply chain and therefore impacts all partner organizations. A typical ecosystem for a set of therapies from one company will include the following actors (organization units):
- collection centers
- treatment centers
- shipping partners
- control tower (which takes orders, coordinates delivery across all the partners, and releases the product)
- product manufacturing
- quality control.
To illustrate the complexity, Figure 2 shows examples of some of the personas in different organizations involved in the COC/COI and their different perspectives on traceability. It is important to consider the range of simultaneous activities people are undertaking while controlling COC/COI because cognitive load is a key factor in the human errors we must avoid.
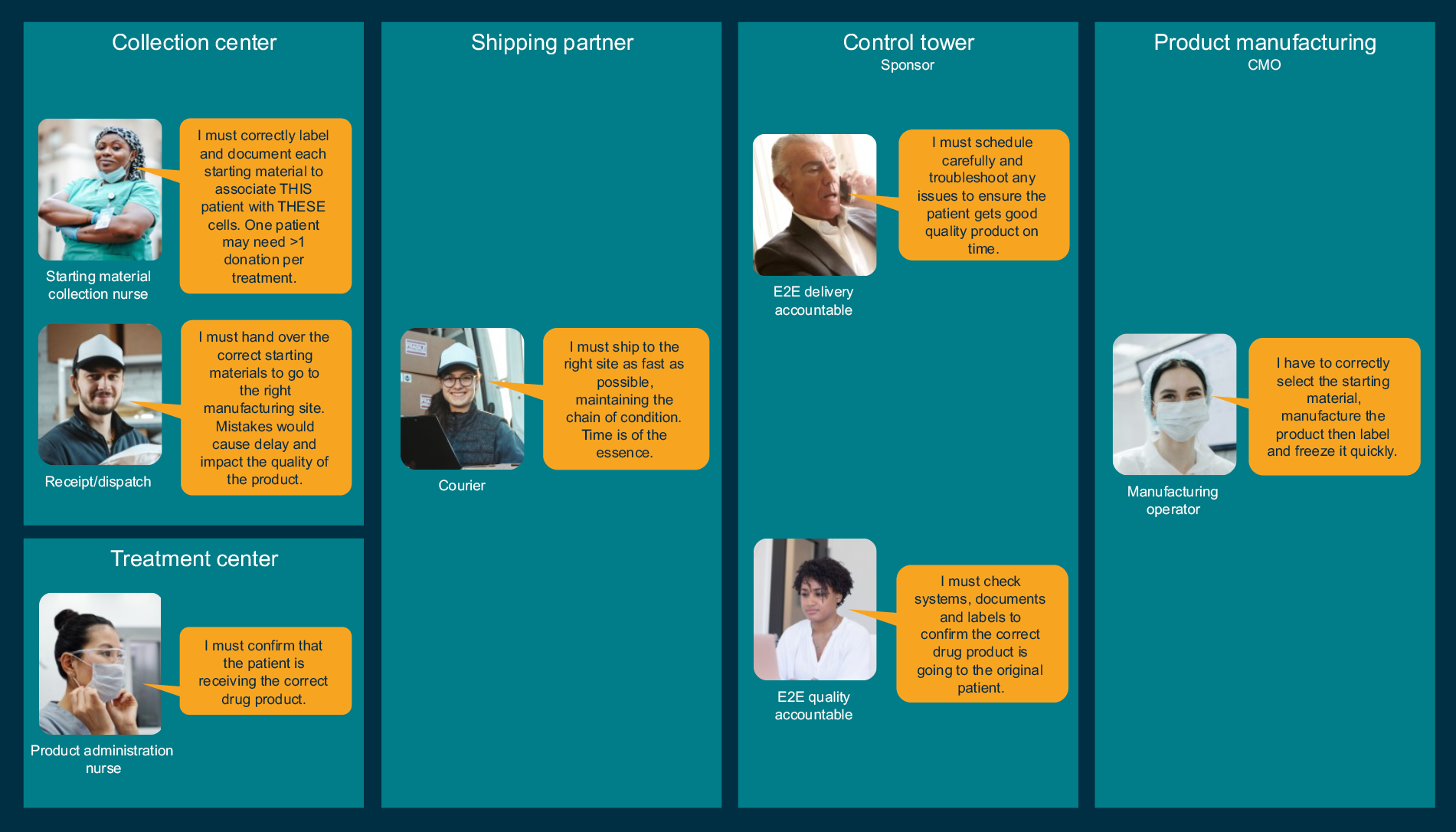
Figure 2: Some personas in the supply chain for a personalized therapy. Click on image to enlarge.
Any solution for traceability must help the users, not make their tasks harder. A truly user-centric design must start with a deep contextual understanding of the people and what they are trying to achieve; otherwise, a system intended to mitigate risk actually increases the chances of human error.
Process Flows
A cursory examination of the new product pipeline of many large manufacturers indicates that most aim to provide a range of personalized therapies. For this to scale up and achieve efficiency overall, it is important to avoid a proliferation of different systems and approaches for each type of therapy. Our aim is to model a consistent set of process blocks and supporting services that can be adapted to any type of personalized therapy.
We use a model process flow (see Figure 3) to show the flow of activity, physical materials, and data within and between these actor organizations to deliver therapy to a patient. This is the flow that must meet the requirements for traceability, so this perspective emphasizes the steps that have a traceability requirement and shows as separate blocks the steps where different systems are typically used.
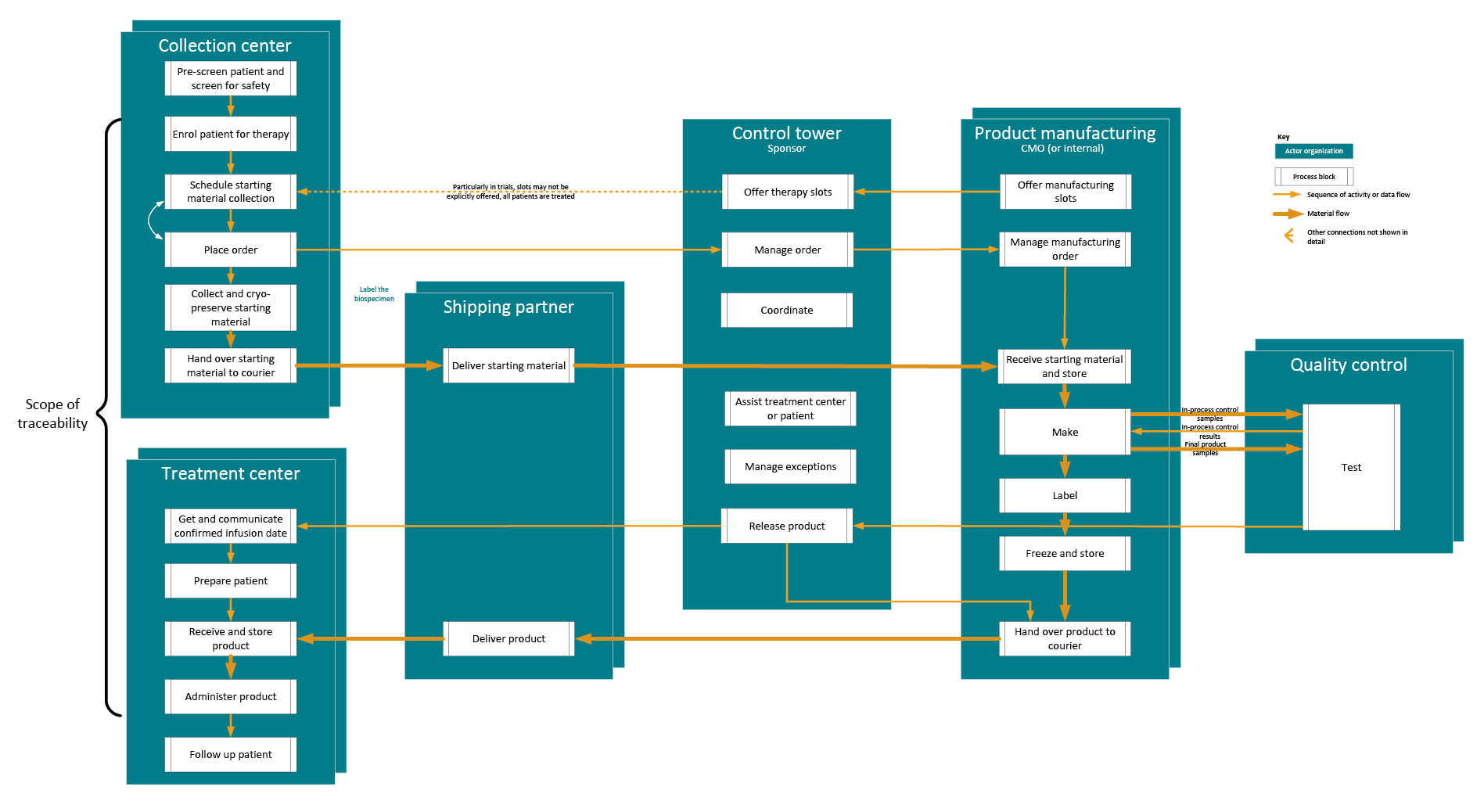
Figure 3: Model process flow for traceability. Click on image to enlarge.
Vision User Stories
Our previous publication, CGT personas and user stories, listed 65 user stories that are distinctive to the supply of CGTs, for a range of personas in the end-to-end CGT process. There are five key personas that relate to traceability:
- healthcare professional
- treatment center coordinator
- end-to-end delivery accountable
- end-to-end quality accountable
- manufacturing operator.
The high-level user stories for these personas can be used as a starting point to accelerate a traceability compliance analysis (see Figure 4 as an example).

Figure 4: User story for a healthcare professional (extract only). Click on image to enlarge.
The Benefits Of Better Traceability
Better traceability is for a purpose: to enable business improvements that bring benefits that support the strategic objectives of organizations and improve the lives of patients. Figure 5 considers how the benefits accumulate as the solution used for COC/COI becomes fully automated and reliable, and then as it becomes standardized.
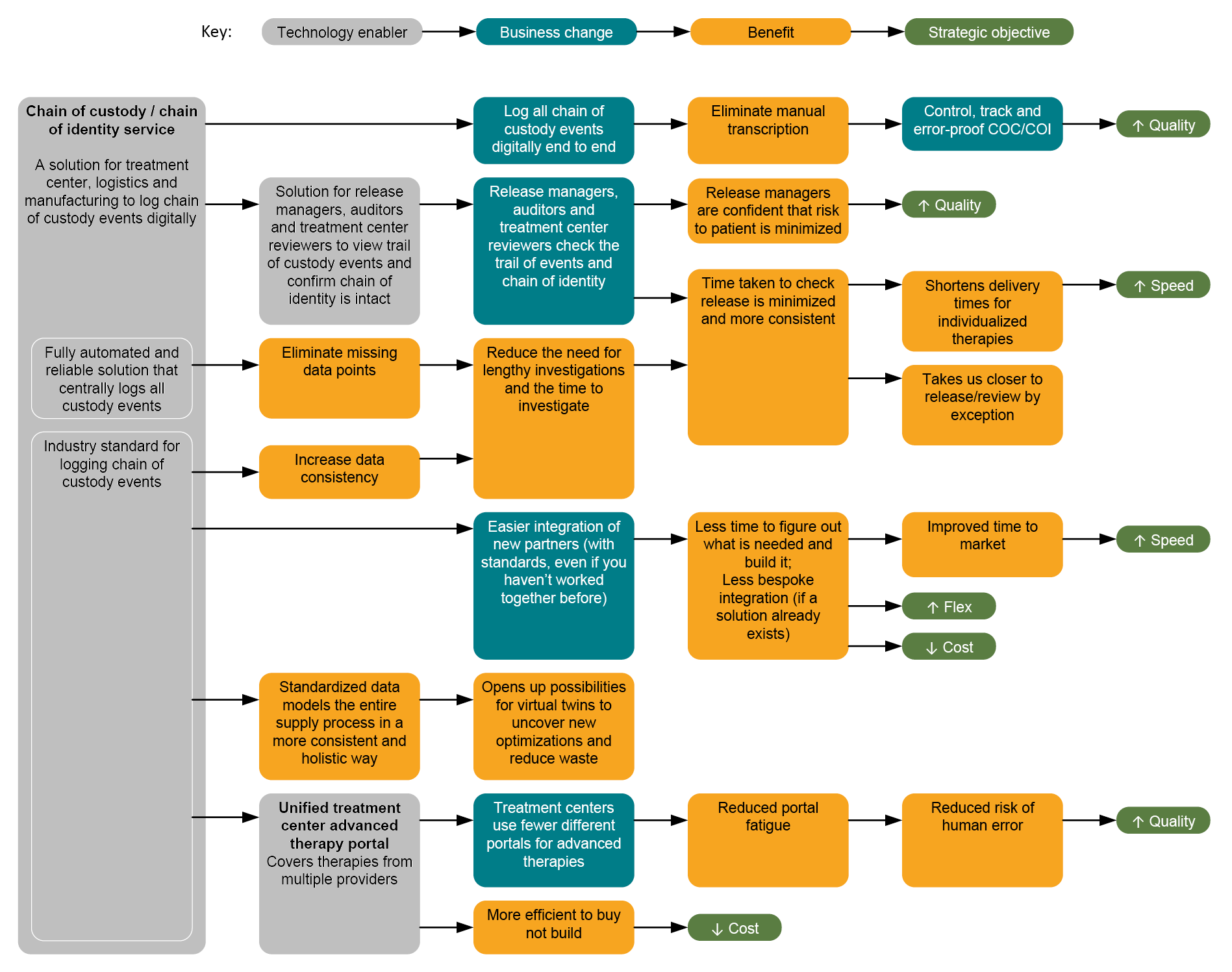
Figure 5: Benefits of a chain of custody/chain of identity service. Click on image to enlarge.
By logging all COC events digitally and end-to-end, data will be more consistent and less likely to have errors that can occur with manual transcription. The goal is to have COC/COI information controlled, tracked, and error-proof.
With a digital solution for COC/COI, release managers, auditors, and treatment center reviewers can view the trail of custody events and confirm the COI is intact. The time to review information leading to release is optimized and more consistent.
A fully automated and reliable COC solution logs all custody events centrally, so missing data points are eliminated. This reduces the need for lengthy investigations and the time to investigate, minimizes the time taken to check release, and makes it more consistent. This approach ultimately shortens delivery times for individualized therapies and brings the process closer to review and release by exception.
Traceability As A Set Of Services
We can model traceability as a set of services or packaged business components. This helps to make sense of the huge variation in how these services are currently implemented: sometimes as part of an existing platform, sometimes on a new platform for CGT, and sometimes as custom microservices.
People in multiple actor organizations must collaborate to supply a personalized CGT. To collaborate digitally, they need to use the systems available in their separate organizations to interact with common traceability services. Treatment centers, couriers, contract manufacturers, and sponsor organizations all need to interact with the same services, potentially from different user interfaces.
Most of the actor organizations are dealing with multiple therapies with different partners. It would make sense for digital interactions to work consistently across all of them rather than creating custom integrations each time. Having services that work in the same way and use the same terminology, process steps, and data points also facilitates the goal of having a common treatment center advanced therapy portal that can handle therapies from several different providers, accessed using standard application programming interfaces.
For rapid start-up, a basic custom service can be established quickly, developed to offer additional features later, or swapped out for a more sophisticated solution when a new enhanced service becomes available.
For some geographical areas, some types of data (particularly patient information) must be held on servers. It makes sense to encapsulate this challenge as a service and hide the complexity of implementing a geographically distributed database from the other functions that use that data.
Applying the principles of modeling as services to the process flow for personalized therapies, a simple overview in Figure 6 shows the services that should support the main process blocks identified above.
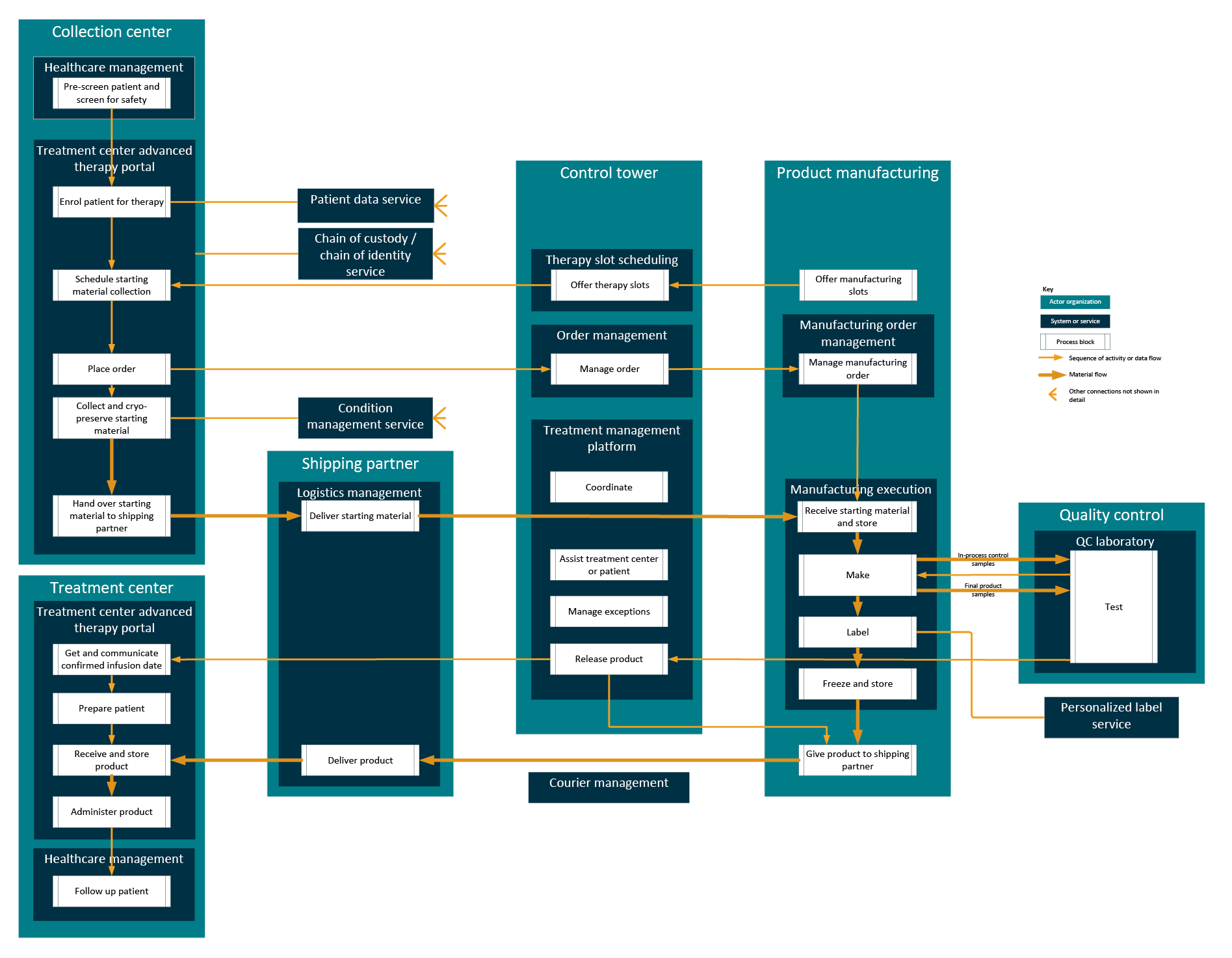
Figure 6: Services required to support the process digitally. Click on image to enlarge.
Traceability As A Set Of Identifiers
Even in a simplified model, many identifiers are used throughout the supply process. In most cases, global standards do not yet exist for identifiers in personalized medicine, so each type of identifier must be chosen to ensure there is no possibility of mix-up, fully considering that a typical configuration involves systems of different types in several organizations.
But even with carefully chosen identifiers, it has never been clear which identifiers should be used to meet the requirements for traceability. Practice varies, with most stakeholders relying on the identifier they are most familiar with. This eases the initial implementation but can make it difficult to work with multiple partners who have taken different approaches.
Standardizing identifiers paves the way for automating verification, reducing manual transcription, and having fewer and more standard bar codes. Today, the bar code may be the primary way of entering data into a system. In the future, it should just be used to verify data that is already joined up throughout the patient treatment journey.
We want to see industry converge on a common approach to identifiers to simplify the challenges of interoperating across partners. Linking the identifiers through the supply chain systems, documents, and labels makes it possible to demonstrate that each personalized treatment is administered to the correct patient. Other data fields will be important at each stage, but a few “keys” link all the stages and provide traceability for the COI:
- A unique pairing of patient ID and treatment ID triggers the creation of a globally unique chain of identity ID.
- The chain of identity ID can then be associated with a unique donation identifier from starting material collection to receipt at the manufacturer, or a batch ID, from first processing to treatment administration.
By following this approach, designers will become clearer on key design choices relating to traceability for initial implementations and able to see the consequences for scaling up the solution to support multiple therapies and many partners. Traceability can be assured for the long term, a wide range of therapy types can be supported, start-up will be faster, and it will become possible to scale up therapy production with easier onboarding of a wider range of treatment centers and manufacturing partners.
Figure 7 adds to the services diagram (Figure 6) the main identifiers we would expect for each data object those services manage. When multiple partners take similar roles in parallel in a scaled-up ecosystem, we must consider how global uniqueness is maintained.
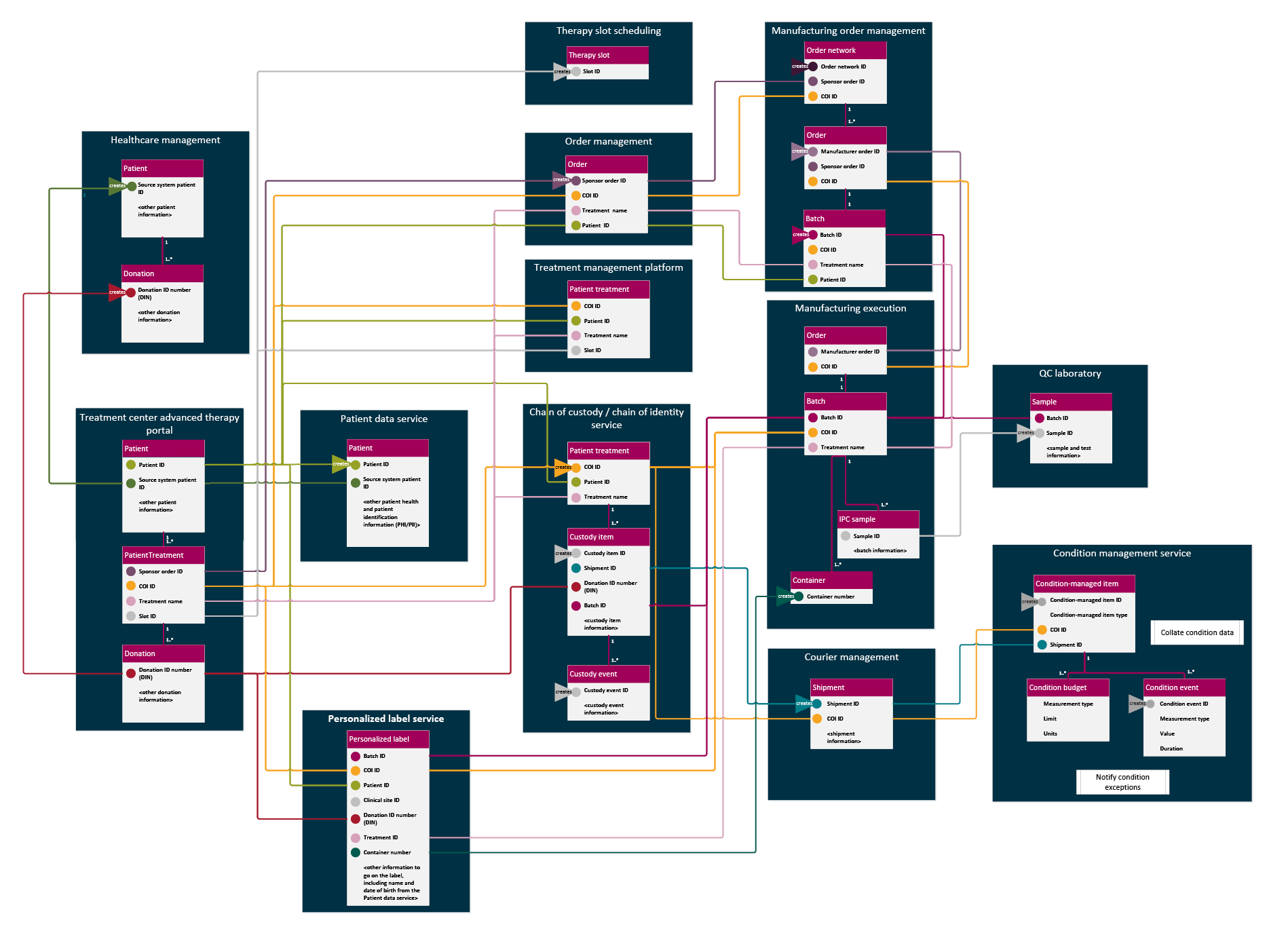
Figure 7: Identifiers used by services in personalized CGT supply. Click on image to enlarge.
Conclusion
Traceability for personalized CGTs has not yet reached digital maturity. There are many approaches but little standardization across CGT license holders. This means that treatment centers and contract organizations must remember all the subtle differences and deal with unnecessary complexity. As the number of approved CGTs grows, the propensity for human error will also increase unless there is standardization.
Using and adhering to the standards outlined will accelerate the analysis of the business challenges and solution designs, highlight key issues to be resolved, and make it easier to align and partner with others working on similar or connected solutions.
Taking this approach will help standardize the traceability of personalized therapies, automate enforcement and review of traceability data, and enable robust end-to-end integration. Once traceability is automated and robust, product release will be faster and anomalies will be resolved more quickly, meeting tight turnaround times and improving patient outcomes.
This article is a summary of a recent BioPhorum publication on the topic. To read more, check out the full paper in Standardizing traceability of personalized cell and gene therapies.
