A Novel Approach To Managing Risks In Aseptic Processing Of Cell & Gene Therapies
By Darius Pillsbury and Tiffany Baker, ValSource, Inc.

Establishing a robust contamination control strategy (CCS) for advanced therapy medicinal products (ATMPs), also referred to as cellular and gene therapy products, is of utmost importance to ensure consistent supply of drug product that is safe for patients and meets product quality requirements to support all stages of the product life cycle, including clinical development and, ultimately, commercial purposes. As most ATMPs are required to be sterile, aseptic processing is required at many steps of the manufacturing process. These manufacturing processes present unique and different challenges from processes used for the manufacture of better understood biological and pharmaceutical product types.
Many novel manufacturing processes that are in early development have been transferred from an academic or clinical setting into a cGMP compliant site for the purposes of the manufacture of clinical trial material (drug product). These processes typically require significant optimization, especially with regard to improving the overall rigor of the controls for consistent sterility assurance. As many of these processes cannot be terminally sterilized (e.g., for the manufacture of a cell therapy or gene-modified cell therapy), aseptic processing controls throughout the manufacture of the product over multiple unit operations and extended durations of time are required. Whereas traditional biological parenteral drug product sterility assessments focus on filling and sealing vials (although that is also in scope of the methodology proposed herein), these assessments can be applied to aseptic processes that span the multiple unique unit operations for various different types of ATMP manufacturing processes.
As part of initially establishing and then maintaining the CCS through the product life cycle, risk assessments and risk-based approaches must be employed to identify and manage the risks of aseptic processing. These requirements can be found in many regulatory requirements and harmonized guidelines, including:
- Risk management is applied in the development and maintenance of the CCS to identify, assess, reduce/eliminate (where applicable), and control contamination risks.1
- Processes, equipment, facilities, and manufacturing activities should be managed in accordance with quality risk management (QRM) principles to provide a proactive means of identifying, scientifically evaluating, and controlling potential risks to quality.
- QRM principles, as detailed in ICH Q9(R1) and EU Guidelines in Annex 20, are particularly important for this class of medicinal products and should be used to develop the control strategy across all stages of the development and manufacturing steps to minimize variability and reduce the opportunity for contamination and cross-contamination.2
- Any intervention or stoppage during an aseptic process can increase the risk of contamination. The design of equipment used in aseptic processing should limit the number and complexity of aseptic interventions by personnel.3
In this article, a formal, systematic approach to managing the unique risks of aseptic processing of ATMPs, the aseptic risk evaluation model (AREM), is presented. The AREM utilizes the collective product knowledge and process understanding and is compliant with the evolving global regulatory landscape and based on industry best practices.
What Is The AREM?
The novelty and complexity of these unique manufacturing processes pose a distinct challenge when it comes to assessing the risks related to process and contamination control. The risk identification, analysis, and evaluation steps of the risk assessment process thus can be difficult when limited knowledge is available from process development (especially when there is limited manufacturing experience in the cGMP-controlled environment). When evaluating aseptic processes for ATMPs, as risk facilitators, we found too much subjectivity in the currently available and best-known methodologies. We were hearing from the risk team “I think this should be the score” (indicating an attempt to score future controls rather than current state) or “we have not done that yet but it should be fine” (indicating a significant potential for bias in the absence of data and higher than normal risk tolerances in some organizations) that really pointed to a need for a new approach to these assessments as the use of ATMPs and new modalities is rapidly expanding.
A modified version of the intervention risk evaluation method (IREM)4 has been developed to better address these specific and unique aseptic processing steps that are used in the production of an ATMP. This risk-based approach employs critical thinking by subject matter experts to make better informed decisions. Thus, we needed a methodology more appropriate for assessing these types of risks that is objective, logical, and) easy to understand and use.
Risk is defined in ICH Q9 as the combination of the probability of occurrence of harm and the severity of that harm. In some instances, risk assessment approaches require assessment of parameters other than likelihood and severity to better understand the risk of the hazard. These are referred to throughout risk guidance and regulation as risk-based approaches. In this instance, the “harm” of the aseptic manipulation is defined as microbial contamination of the product. When identifying risks in aseptic processing, the severity of that specific potential harm is thus always considered to be “high”. For this reason, the AREM tool (like its predecessor, the IREM) is designed to focus on parameters specific to the performance of aseptic manipulations and how they might result in microbial contamination of that manufacturing process. When determining the inherent risk of an aseptic manipulation in relation to all other manipulations performed, there are a few more factors better suited to help us understand the risk – duration, complexity, and proximity, as shown in Figure 1. We will discuss each in more detail below.

Figure 1: Factors Evaluated for the Determination of Overall Risk to Aseptic Processing
The Aseptic Risk Evaluation Model Process
As with any quality risk assessment, the process begins at the risk initiation stage of the life cycle by defining the risk question, which in this case can be captured as simply as:
“What is the relative risk of loss of sterility (sterility assurance) from each individual manipulation performed within the aseptic boundary during manufacture of the drug product (ATMP)?”
Once your risk question is refined and thoroughly understood, the risk tool and methodology best suited to answer the question can be selected. A risk question like the one above is a perfect fit for the AREM methodology, and the simple process to complete this risk-based approach can be broken down into the following steps:
 Figure 2: Steps in the Aseptic Risk Evaluation Model (AREM)
Figure 2: Steps in the Aseptic Risk Evaluation Model (AREM)
Step 1: Risk-Based Approach Pre-work
A risk assessment, or in this case a risk-based approach, is only ever as good as the information used to inform it. It is critical to ensure the appropriate stakeholders are included in the process. The AREM SME team needs to include representatives of multiple departments and, most importantly, those personnel who perform the aseptic process in the GMP manufacturing setting. An example SME team could be composed of MSAT, manufacturing, quality assurance, QC microbiology, and sterility assurance. Additionally, a risk facilitator should lead the effort, someone who has been trained both in QRM principles as well as specific AREM tool methodology.
Once the team has been selected, the preparation phase of the AREM can begin. Scope setting is an important step for the AREM, which is where the working team will define the boundaries of the aseptic manufacturing process – where the scope for the assessment will begin and where it will end. Generally, the AREM will involve the manipulation for any step where the product flow path or in-process material is exposed to the environment or where the product flow path is broken in any way. Discussions should be had as to whether process steps like sterile welding of the flow path is in or out of scope and clearly captured as an assumption for the effort. Additional examples of process steps or unit operations that can be included within the scope of an AREM are captured below:
Examples of process steps/unit operations that can be assessed using the AREM:
- Manual operations performed in open system (BSC within a Grade B cleanroom) ⇨ manual operations performed in a closed system (incubator within a Grade C cleanroom)
- Aseptic manual connections to prepare process solution (cultivation media) – filtration by 0.2 µm filter of cultivation media
- Use of “off-the-shelf” medical tubing and connectors – custom designed and sterilized assemblies
- Identify need for in-process microbiological testing (e.g., bioburden, sterility) post unit operations with a series of high-risk process steps.
Lastly, the AREM working team should gather important information to input into the working session. One of the most helpful activities to perform during this stage is the process demonstration. Demonstration of the aseptic manipulations by the experienced personnel is important for the entire team to understand and thus to better score the risk factors. This can be done by simulating the aseptic processing steps in a development laboratory or even in a conference room, e.g., performing the aseptic manipulations using water in place of the product and process solutions. If available, video of these aseptic manipulations from the actual manufacturing areas also can be informative.
Step 2: Determine All Aseptic Steps Performed within the Aseptic Boundary
The first activity to be performed once working sessions begin should be the identification of all aseptic steps within scope to be assessed using the tool. The working team should perform this task by reviewing each batch record (if available), step by step, to determine each individual aseptic manipulation performed. The demonstration of the process that was performed during the pre-work phase also can help determine this list.
Step 3: Rate Each Individual Manipulation for Each Factor
Each aseptic manipulation, once identified, will need to be scored by the risk team to determine the relative risk of one manipulation in relation to all others performed for the process. All aseptic manipulations for a given process will be scored using the same criteria, one that the working team should agree on prior to starting the assessment. The factors that were considered for the overall risk of an aseptic manipulation were individual aspects of that manipulation that had the capability to increase the likelihood of a contamination event or one that would amplify the consequence on the product or intermediate if a contaminant were to be introduced. The factors considered critical in determining the overall risk of an aseptic manipulation can be found below in Table 1 and the individual rating criteria in Table 2.
 Table 1: Aseptic Manipulation Factors Considered for AREM
Table 1: Aseptic Manipulation Factors Considered for AREM

Table 2: Risk Ranking for Factors Used in the IREM
Step 4: Determine Overall Risk Score for Each Manipulation Using AREM Matrices
The working team will be able to determine the overall risk for each individual aseptic manipulation by inputting the risk factor scores into the AREM methodology. Three-factor scoring necessitates a two-matrix approach; the first matrix combines the complexity and duration ratings, producing a preliminary risk value (in essence a place holder), which is input into the final matrix alongside the proximity rating to determine the overall risk value. The preliminary AREM matrix used to determine the preliminary risk value is shown in Table 3 below:
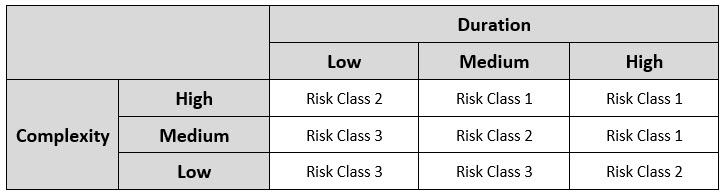
Table 3: Preliminary Matrix Used in Determination of Initial Risk Class
A final risk matrix combines the preliminary risk value for each aseptic manipulation with its proximity rating to determine the overall risk value for the manipulation. The final risk matrix can be seen below in Table 4.
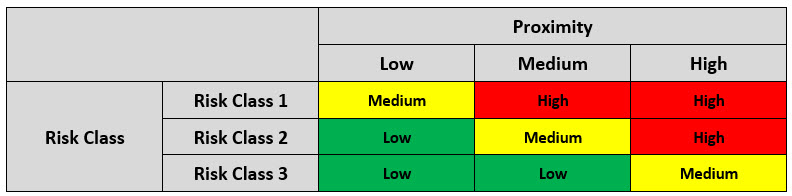
Table 4: Final Matrix for Determination of the Overall Risk Score
Upon the determination of the overall risk score, the aseptic procedures are ranked by risk and specified actions are to be taken, as shown in Table 4.
Step 5: Determine Risk Acceptability and Risk Mitigation (if needed)
The working team can now use the overall risk score for each individual aseptic manipulation to determine acceptability of each manipulation in terms of risk (e.g., are they appropriate to perform in the process, do they need to be improved in order to continue, etc.). To do that, a risk acceptability table must be employed consistently and uniformly across all rated manipulations. See Table 5 below for a suggested AREM risk acceptability table.
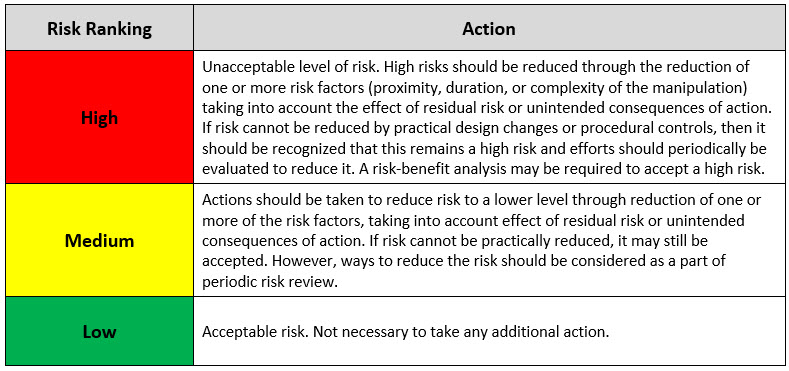
Table 5: Risk Acceptability Table Based on Overall Risk Score
Step 6: Outputs of the AREM – Document the Assessment and Strategic Outputs
The output of the AREM is incredibly useful and can be leveraged in a number of circumstances, as shown in Figure 3.

Figure 3: Utilization of the Outputs from the AREM Assessment
Conclusion
The AREM risk assessment tool should be managed through the life cycle of the manufacturing process. It is recognized that not all aseptic manipulations of higher relative risk can be mitigated for immediate use in GMP manufacturing and that further development and design of these aseptic processing steps can take time and resources. For this reason, the higher risk steps must be the focus of aseptic training and acceptable performance by each of the personnel who will be performing these aseptic manipulations must be demonstrated.
In article 2 in this series on the topic of the AREM, we will provide a life cycle approach for the use of the outputs of the AREM as well as the inputs required to ensure the assessment is always a snapshot of the current state of your manufacturing process. Article 3 in the series will include a case study on how the AREM can be applied to an aseptic process that is used in the manufacture of a gene-modified cell therapy.
References
- EudraLex Volume 4: EU Guidelines to Good Manufacturing Practice Medicinal Products for Human and Veterinary Use. Annex 1 Manufacture of Sterile Medicinal Products, European Commission, Brussels, Belgium, 2022: https://health.ec.europa.eu/system/files/2022-08/20220825_gmp-an1_en_0.pdf
- Guide to Good Manufacturing Practice for Medicinal Products Annexes - Pic/s, PIC/S, picscheme.org/docview/6608. Accessed 29 Dec. 2023.
- Guidance for Industry - U.S. Food and Drug Administration - Sterile Drug Products Produced by Aseptic Processing — Current Good Manufacturing Practice, Sept. 2004, www.fda.gov/media/71026/download.
- Baseman, H., and Long, M. “Risk Management of Microbial Contamination Control in Aseptic Processing and Interventions Risk Assessment Model (IREM): The Use of Critical Thinking to Make Informed Decisions.” In Contamination Control in Healthcare Product Manufacturing, Vol. 3, eds. Russell Madsen and Jeanne Moldenhauer, 341-404. Bethesda: PDA/DHI, 2014
About The Authors:

Darius Pillsbury is a senior consultant with ValSource and works with organizations in the development and manufacture of pharmaceuticals and biologics, including advanced therapy medicinal products (i.e., cell and gene therapies), as well as other new modalities. He focuses on developing, implementing, and managing phase-appropriate quality systems and process validation activities through all stages of the product lifecycle. He has extensive experience in the employment of a science-driven and risk-based approach to the establishment of control strategies for manufacturing processes and contamination control in line with industry best practices and compliant with global regulatory requirements and guidance. He currently serves on the PDA Advanced Therapy Medicinal Products Advisory Board and has developed technical reports, PDA/ANSI standards for ATMPs, and is an instructor of process validation for the FDA Compounding Quality Center for Excellence. Pillsbury has a BS in chemical engineering from Tufts University.

Tiffany Baker is a senior consultant at ValSource, LLC, specializing in development and implementation of innovative approaches to quality risk management (QRM), QRM program design, creating a risk-focused culture, and developing risk-based approaches to support contamination control strategies. She is an active member of ISPE and the PDA; a faculty member for PDA’s Training Research Institute; and an instructor for the PDA courses on quality risk management foundations, practical application of QRM tools, and QRM for the design, qualification, and operation of manufacturing systems. She is co-lead for the PDA task force on remote audits and inspections. Additionally, she was an author for the revision to ISPE Baseline Guide 5: Commissioning And Qualification. She has a BS in microbiology from the University of Rhode Island and an MBA from Providence College. She can be reached at tbaker@valsource.com.
