A Holistic Approach To Container Closure Integrity
By BioPhorum
Container closure integrity (CCI) is the ability of a container closure system (CCS) to maintain a sterile barrier against potential contaminants and to prevent leakage out of the CCS. Contaminants that could potentially cross a container closure barrier include microorganisms and reactive gases. A CCS must maintain the sterility and product quality of sterile final pharmaceutical, biological, and vaccine products throughout their shelf life.
The traditional expectation has been to demonstrate control by testing, but current regulations are pushing for a science- and risk-based approach, which means building quality into the design and processes rather than testing/inspecting the manufactured item.
This article outlines best practices for establishing a holistic approach to ensuring CCI. For new programs, this preferred focus starts in development and continues through validation and manufacturing. For existing/approved programs, the holistic approach will have less focus on development and validation by focusing on manufacturing while leveraging knowledge and experience to verify ongoing control documented as detailed in the associated risk assessments. It demonstrates that a holistic approach to CCI can provide a viable alternative to final testing. The sections are set out according to the phase of a product’s life cycle.
Development
CCS Development
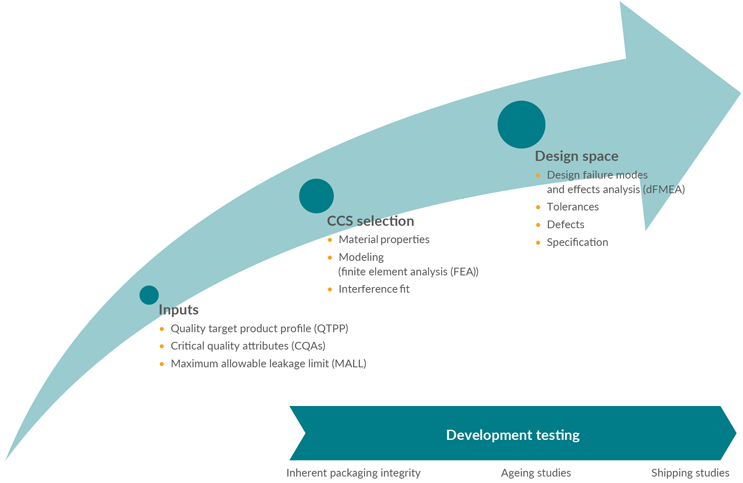
A suitable CCS for the drug product is selected during the development process. The development team identifies how the CCS components collectively achieve CCI and what aspects of the components contribute to tightness in drug product manufacture, transportation, and storage. This is achieved in three successive phases (see Figure 1) and is supported by development data.
Figure 1: Development phases. Click on image to enlarge.
Quality Target Product Profile (QTPP) And CCS Selection
Defining the QTPP is usually the first step in drug product development. Consider key inputs for selecting a suitable CCS and for defining in which conditions the CCI will need to be ensured. The following aspects directly impact CCI:
- shelf life,
- high-stress manufacturing processes (e.g., terminal sterilization),
- storage, shipment, and distribution environments, and
- drug product is integrated with a combination product (additional regulatory framework).
Other aspects indirectly impact the development of a holistic CCI approach. For example, if a barrier coating is needed to mitigate leachables, the development team needs to evaluate the impact of the reduced contact between the rubber closure and the container.
Maximum Allowable Leakage Limit (MALL)
The MALL is the maximum leakage rate tolerable for a given product package that poses no risk to product safety and no (or inconsequential) impact on product quality. Specifically, a loss of CCI could have the following effects:
- entry of microorganisms into the CCS, leading to failure of product sterility,
- escape of the product dosage from or entry of external liquid or solid matter, leading to failure of product physicochemical quality attributes,
- change in gas headspace content, leading to failure of product physicochemical quality attributes, and
- change in headspace vacuum, leading to the hindrance of product access by the end user.
The severity of CCI loss needs to be assessed formally, considering the product’s QTPP.
Initial CCS Characterization
The focus of initial CCS characterization is to identify the technical aspects that may impact the robustness of the system under different conditions. Typically, this is achieved using paper-based analyses, computer models, and exploratory testing. The sample size for exploratory testing is at the engineer’s discretion and should be adjusted as needed during this phase. The goal of this phase is to understand how the component seals the container, especially by identifying the sealing surfaces.
Supplier Knowledge And Controls
Once defects and dimensional requirements have been identified as a result of the initial CCS characterization, defects and dimensional requirements should be reviewed with potential suppliers to ensure they can meet the specified requirements. These component requirements and other expectations should be captured in either the component specification or supplier quality agreements.
Risk Assessment
A risk assessment is executed as part of a CCS development. This exercise is not limited to CCI but integrates CCI-related risks with other essential requirements derived from the QTPP. Regarding CCI, the risk assessment combines learning from the initial characterization and the conditions mentioned in the QTPP. The risk assessment may consider the following aspects:
- material,
- supplier production process,
- final closing process, and
- ICH Q9: Quality Risk Management principles.
Extended Characterization
To ensure a comprehensive development package, all identified risks should be supported by characterization studies to gain a greater understanding of the process and develop a proper control strategy. Extended characterization data directly supports the risk assessment ratings. A sound experimental design and appropriately powered sample sizes are required.
Platform Approach
Whenever a drug product does not impact container integrity and/or functionality, a platform approach with an empty or water-filled CCS can be considered for all development testing. It is known that high pH, preserved formulation, or frozen product can create additional risk. The product may be considered later in the life of the project, which may impact the choice of method.
Design Verification Of Combination Products
For a CCS that is meant for combination products, the extended characterization work will be performed as part of design verification activities. Whenever possible, development activities performed according to quality by design should be leveraged using the holistic control strategy. This is challenging when quality by design activities have not considered the needs of the design control process.
Component Manufacturing And Controls
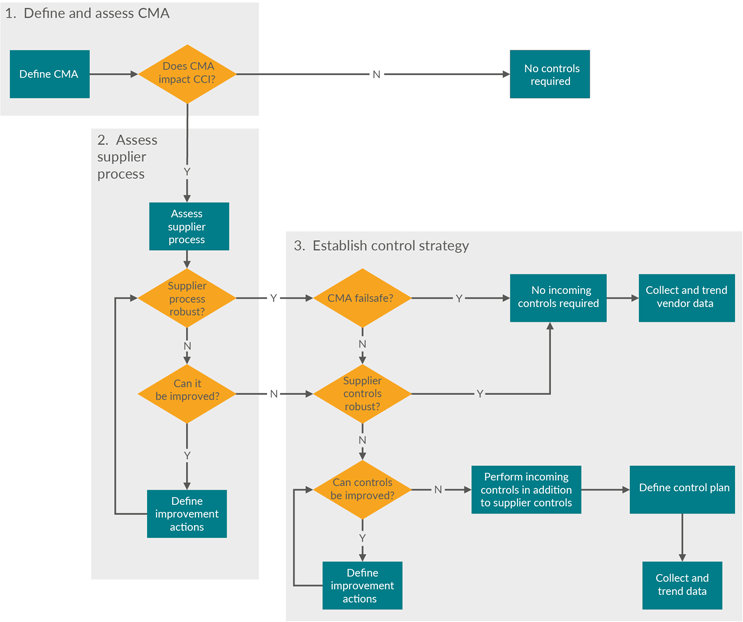
Consider the impact of component manufacturing and processing on overall CCI. Figure 2 is a decision tree to help define the critical material attributes (CMAs) and the supplier control strategy.
Figure 2: Decision tree to select the component manufacturing level of oversight. Click on image to enlarge.
Define CMAs For Assessment Of CCS Critical Attributes
The output of the risk assessment identifies the CMAs to be maintained and controlled. These can be used to assess how supplier processes impact the CMAs. As with all manufacturing processes, the production of primary packaging components includes variability and can result in components that are unfit for use. So, it is critical to understand the primary packaging component supplier processes and their operating ranges to ensure that appropriate preventions and controls are implemented during component manufacturing and supplier release.
Assess Supplier Process — Supplier Preventions And Controls During Manufacturing Of Components
CMAs allow a control strategy to be established when assessing the supplier manufacturing process for potential to harm. This is required to maintain appropriate component quality upon release by the supplier and subsequent incoming acceptance by the biopharmaceutical manufacturer. A CMA is considered a failsafe when, by design, it has a low probability of failing the specification.
Establish Control Strategy
Based on a thorough primary packaging component supplier manufacturing process analysis, a holistic component control strategy can be established and implemented. This can be based on confirmation of a sufficient supplier quality system with preventions and controls and/or additional testing that can be requested in the biopharmaceutical manufacturer’s component release specification. A review of supplier process failure mode and effects analysis (pFMEA) documents should give insight into the basis of the control plan that is in place as well as the degree of planned continuous improvement to improve process robustness.
Validation
According to relevant regulatory guidance, process validation (PV) is divided into three stages over a product life cycle: process design (stage 1), process qualification (stage 2), and continued process verification (stage 3). PV/process performance qualification (PPQ) represents the collection and evaluation of data to establish scientific evidence that a process is capable of consistently delivering products of predefined quality.
The holistic approach to CCI is aligned with the practice above. If this is applied to CCI, the initial process design (stage 1) would involve both initial CCS development and qualification of the component assembly process, including machinability or engineering runs to define the manufacturing process.
Component Assessment
Understanding component variability in CCI and knowledge of CMAs are key outputs from CCS development that can be used to define specifications and implement controls around primary container inputs. These are important elements of an overall CCI validation strategy that should be defined before proceeding to PV/PPQ. The objective of component qualification is to assess how much natural validation in the component manufacturing process will impact CCI.
CPPs For Ensuring CCI And Controls
The critical process steps involved in converting individual packaging components into a fully assembled CCS should be identified before PV/PPQ. These process steps may be performed as part of component preparation or during fill/finish. After the process steps are identified, qualification of the entire CCS assembly process before PV/PPQ is recommended to demonstrate that process targets, equipment setpoints, and operating ranges involved in each process step can reliably produce an integral CCS that meets its critical quality attributes. We recommend that these qualification studies involve challenging the upper and lower limits of a parameter’s operating range to assess the impact on CCI.
Recommendations For Confirming CCI During PV/PPQ
- In general, the goal of PV/PPQ is to confirm the effectiveness of the control strategy and that the expected CCI validation output is scientific evidence that demonstrates a CCS will meet critical quality attributes when assembled during the commercial manufacturing process.
- Controls related to packaging component inputs, equipment, and process parameters are derived from the successful completion of qualification activities and are in place before proceeding to PV/PPQ.
- Ultimately, PV/PPQ should demonstrate CCI with the selected packaging component inputs and the commercial manufacturing process, adequately simulating processing stresses to confirm the effectiveness/robustness of the control strategy.
- Results from a successful aseptic process simulation can be leveraged as additional proof of the CCI performance without the need to perform microbial ingress testing.
- PPQ batches are typically used to confirm the shelf life evaluation initiated in previous studies.
Routine Manufacturing
Manufacturing processes are critical to creating and maintaining CCI. A well-thought-out and implemented integrated control strategy will eliminate the risks of a detrimental impact the manufacturing processes may have on CCI. In a holistic approach, the most prevalent steps of a good manufacturing control strategy are well-designed preventions. If preventions cannot eliminate all sources of risk to CCI by themselves, they should be backed up by good in-process controls. Any post-manufacturing testing can only be a high-level confirmation that the manufacturing processes are under control. Post-manufacturing testing cannot be proof of good product quality.
Shipping And Stability
Under a holistic CCI approach for drug products, most of the factors affecting CCI have been incorporated into the development of the CCS and a robust and consistent product manufacturing process. An additional element of this approach is the demonstration of CCI during the entire product shelf life until the point of use to demonstrate that the sterile product is free from microbial contamination. Once the final packaged configuration is identified, a transportation/shipping study is commonly executed as a one-time exercise during product development as part of the submission.
Ongoing Monitoring Of Holistic CCI Strategy
The above text outlines how a thorough holistic strategy ensuring CCI of CCS can be established. The effort should not be seen as a one-time exercise; establishing a robust monitoring and continuous improvement process is equally important. The recommended practice is to ensure technical considerations are embedded in the pharmaceutical quality management system.
Conclusion
Applying a holistic approach to CCI for parenteral drug products is well aligned with the principles of quality by design. One of the critical functions of a suitable CCS is to provide an integral container that will protect the drug product according to its QTPP throughout its life cycle and prevent leakage out of the CCS. To our knowledge, this is the first industrywide view of a holistic approach to CCI for a parenteral product. It describes how to implement quality within the product life cycle rather than relying on final product release and stability testing. This integration is built on formal risk assessments covering the CCS design and development, validation, routine manufacture, stability, and shipment of the drug product.
This article summarizes a recent BioPhorum publication on the topic. To read more, check out the full, free-to-download paper in BioPhorum’s holistic approach to container closure integrity.