A 19-Color Multiparameter White Blood Cell Panel Designed For The Immunophenotyping Of Normal And Malignant Leukocytes By Flow Cytometry
By Michelle Scott, Chris Brampton PhD, and Kelly Kroeger PhD, Bio-Rad Laboratories

Abstract
Flow cytometry is an important tool for the diagnosis and classification of leukemia. While many leukocyte markers have been identified and are used to determine the exact subtypes of leukemia a patient may have, high-dimensional multicolor analysis has been limited by instrument capabilities. This has often resulted in splitting a valuable limited sample into multiple independent tests to fully define the patient’s phenotype.
A 19-antibody white blood cell panel was developed using the ZE5™ Cell Analyzer from Bio-Rad Laboratories. With up to five lasers and 30 analysis parameters (of which 27 channels are for fluorescence detection), all the markers necessary to classify chronic/acute lymphoproliferative leukemia disorders can be combined into a single tube. Using this panel, a patient with chronic B-cell lymphocytic leukemia was successfully identified from a normal PBMC sample.
Introduction
With the advent of flow cytometry into the clinical arena as a diagnostic tool, high-dimensional multicolor analysis has become increasingly recognized as invaluable for the diagnosis and classification of leukemia. In the past, clinicians were restricted by the number of channels available to construct multicolor panels of leukocyte markers. As a result, defining populations of normal and aberrant blood cells often required splitting valuable samples into multiple tubes to achieve the needed resolution.
Click Here For More Information on Flow Cytometry
The ZE5™ Cell Analyzer, when configured with five lasers and 27 channels of fluorescence detection, allows all the markers necessary to classify chronic/acute lymphoproliferative leukemia disorders (CLL/ALL) to be mixed into a single tube. This panel combines 19 surface membrane markers of both cluster of differentiation and Ig species with TCR expression to successfully dissect aberrant leukocyte populations present in a patient with chronic B-cell lymphocytic leukemia (B-CLL).
Materials and Methods
Antibodies
CD3 BUV496, CD4 BUV661, CD19 BUV737, TCRgd BUV395, CD8 BV711, CD45 VioGreen, CD56 BV785, Kappa Light Chain BV421, CD7-BV650, HLADR VioBlue, CD117 BV605, CD33 FITC, CD34 PE-Vio770, CD64 PE-CF594, CD11b PE, CD14 PE-Cy5, CD20 APC-Vio770, CD38 APC-R700, Lambda Light Chain APC
Blocking Buffer
PBS + 50 μg/ml mouse, rat, rabbit IgG + 1% BSA + 1% glucose + 20 mM EDTA + 0.1% azide
Washing Buffer
PBS + 1% BSA + 1% glucose + 20 mM EDTA + 0.1% azide
Beads
AbC Total Antibody Compensation Bead Kit (Thermo Fisher Scientific)
Media
RPMI 1640 or DMEM + 10% FBS
Cell Preparation
Normal PBMCs and B-CLL patient PBMCs were thawed and stained using standard procedures. Thawed samples were placed into 5 ml of prewarmed cell media in 50 ml polypropylene centrifuge tubes. Cells were counted and assessed for viability using the TC20™ Automated Cell Counter (Bio-Rad Laboratories) to obtain an accurate cell count and viability measurement for each sample. PBMCs were allowed to recover in culture media in a 37°C, 5% CO2 incubator for at least an hour.
Cell Staining
The normal PBMC sample and the B-CLL PBMC patient sample (each sample contained 1 x 106 cells ) were treated with blocking buffer for 10 min and then stained by adding a prepared cocktail containing all 19 antibodies. Antibody concentrations used were based on manufacturer recommendations. Samples were incubated on ice, protected from light, for 45 min to an hour. Cells were washed twice and resuspended into 1 ml of ice cold wash buffer. They were stored on ice and protected from light until acquisition on the ZE5 Cell Analyzer. Gating was carried out using FlowJo Software (Tree Star, Inc.). Stained compensation beads were used as single-stained controls (data not shown).
Click here to Meet the ZE5™ Cell Analyzer from Bio-Rad
Results
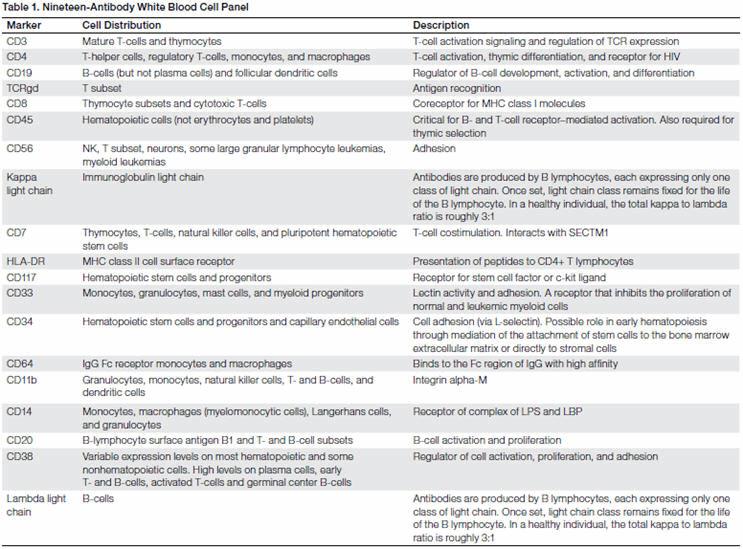
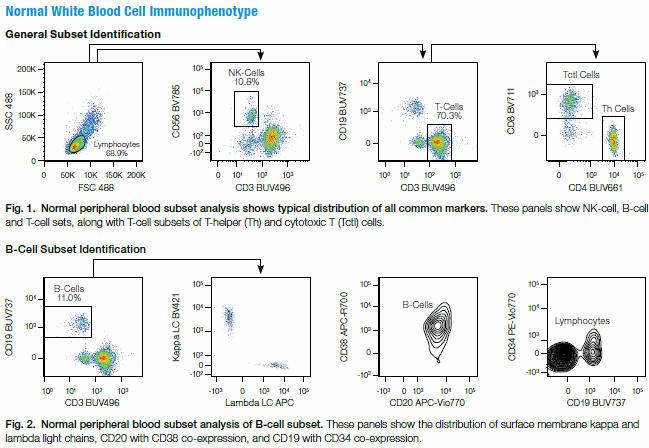
PBMCs from a healthy normal donor were stained using a 19-color panel and run on the ZE5 Cell Analyzer. Results demonstrated that all of the common markers were easily identifiable with no need for FMO controls.
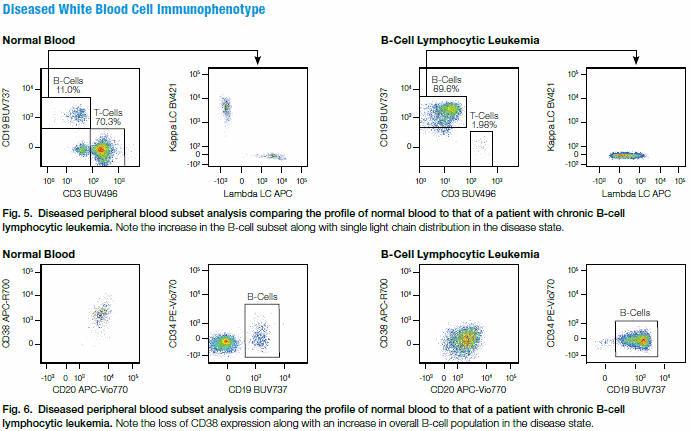
PBMCs from a chronic lymphocytic leukemia patient were then stained with the same 19-color panel. Though the majority of cell populations remained unchanged in this panel, they could be useful for identification of other leukemia subtypes in additional samples. The panel easily identified the patient sample as a chronic B-cell leukemia based on:
- The expression of CD19 along with an aberrant expression of surface kappa light chain and lambda light chain, indicating a monoclonal B-cell population (100% expression of only one type light chain).
- Lowered expression of CD38 on CD20-expressing cells.

Conclusions
- Large-panel multiparameter design and analysis is straightforward using the ZE5 Cell Analyzer’s 27 fluorescent detection channels
- A 19-color panel, designed from existing literature, could allow classification of CLL/ALL disorders utilizing a single tube with the combined antibodies on the ZE5 Cell Analyzer
