A cGMP-Ready Purification Process For Adenovirus Purification
By Mark Fitchmun, Somatek Inc.; Mark Snyder, Bio-Rad Laboratories, Inc.; John Chicca, Molecular Disgnostic Services, Inc.
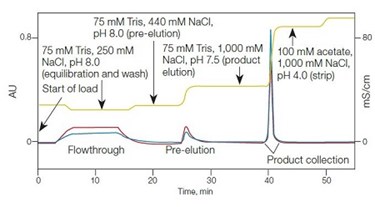
Large-scale downstream processing of viruses for clinical applications poses challenges different from those of many biotherapeutics. These challenges mostly arise from the size and complexity of the virus. For example, he adenovirus vector comprises 25% of all gene therapy clinical trials due, in part, to its ability to infect both nondividing and dividing cells with persistent expression. The vector contains over 2,700 protein subunits and has a mass of approximately 165 MDa and a diameter of approximately 0.1µm.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Bioprocess Online? Subscribe today.
