Supply Chain Risk Where You Least Expect It — Good Supply Practices (GSPs) For The Lifesciences

By Marla Phillips, Director, Xavier Health
During the 2010 and 2011 FDA/Xavier University pharmaceutical/biotech and medical device conferences, members of both industries expressed repeated concerns about the inability to reliably and consistently ensure the supply of incoming materials used in their products. These industries have felt the sting of the heparin case and have recognized that it quite literally could happen to any company at any time. No organization could possibly have the resources to manage their supply chains directly back through their supplier’s supplier’s supplier’s suppliers, and who would have thought to check operations at the pig farm? Our industries put their patient’s safety as their foremost priority, so not having 100% verification of every operation is unsettling at the least.
In response to these concerns, Xavier University formed a team of FDA officials and industry professionals in 2012 to investigate root causes and develop solutions using the DMADV (define, measure, analyze, design, verify) process. (Current team members are listed in Appendix I). Since that time, the team has determined the source of dysfunction affecting the integrity of incoming material and contracted services procured to support the manufacture of finished drugs, biopharmaceuticals, and medical devices. The group has also begun implementing sustainable solutions that can be tied to return on investment — such as increased safety, improved quality, and enhanced reliability — commensurate with the need.
This article will discuss the team’s findings and recommendations for good supply practices (GSPs) through a methodical supply-by-design process that increases the reliability of incoming supply, and therefore reduces the risk to finished product quality, patient safety, and business success.
An Unexpected Source Of Supply Chain Failures
Many in the life sciences industry have long believed, and continue to believe, that the lack of reliability of incoming material is based on poor performance of suppliers. As a result, many supply chain management practices focus on the supplier side of the equation. In order to identify true root causes of supply chain issues, the life science manufacturers on the Xavier team developed a comprehensive list of failure modes associated with the defined process flow shown in Figure 1.
Figure 1: Supply Chain Process Flow

Through the failure mode analysis work, the group recognized a major flaw in their understanding of life science supply chain problems. The failure modes identified were not solely related to poor performance of suppliers, as originally surmised. Rather, all of the failure modes were related to risks that were induced by the manufacturers themselves, or could have been avoided by the manufacturers. This critical paradigm shift has enabled the Xavier team to focus solutions on reducing risk caused by the manufacturers, and therefore reduce risk to the final product and patient
Figure 2: Major Paradigm Shift revealed through Failure Mode Analysis

The identified failure modes were then incorporated into a cause and effect matrix that each manufacturer and FDA officials scored against six critical customer criteria (reliable/safe materials, protect company brand/assets, full supply chain knowledge and oversight, cost effective, supplier awareness of role/responsibilities in the supply chain, and visibility of supplier information across the industries). A survey was then sent to 162 material providers that supply the pharmaceutical, medical device, and food industries. In every case, the suppliers corroborated the findings of the manufacturers’ root cause analysis and, importantly, did not identify any obvious failures missed by the manufacturers.
The direct impact of this paradigm shift is that it:
- Shifts resources from trying to improve supplier performance to improving internal performance that affects the ability of the suppliers to perform well
- Enables industry to take ownership of the failure modes impacting supply chain integrity
- Enables industry to develop solutions that will reduce risk to product quality
- Enables global regulators to understand critical root causes to supply chain integrity and therefore product quality risk
The paradigm shift has played an important role in developing solutions that will actually make a difference. Far too often, industry working groups spend an exorbitant amount of time to develop white papers and guidances that do not: (1) address true root causes, (2) recognize the real world variability and maturity differences from company to company, (3) are not commensurate with the need, and (4) are not actionable solutions that can be implemented in a meaningful way. The Xavier team is developing solutions that companies of all sizes can adopt in a way that is appropriate for their organization, and the complexity and criticality of their operations.
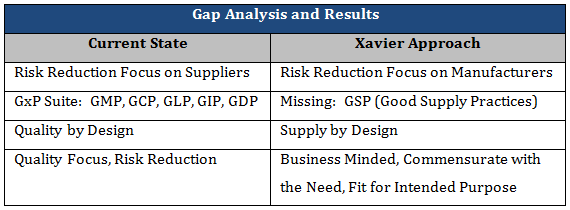
Table 1 details some examples of work that is being done in industry today, versus the gaps being addressed through the Xavier Integrity of Supply initiative. As previously explained, industry is mostly focused on reducing supply chain risk through implementation of supplier controls and oversight. However, the gaps identified by manufacturers pointed to their own organizations, thus indicating the need for risk reduction at the manufacturer. Although there are several GxP’s, an obvious area not addressed is the supply chain. Through the Integrity of Supply initiative, the Xavier team is developing the Good Supply Practices to compliment and complete the remaining GxP’s. Additionally, the pharmaceutical industry has published guidance on Quality by Design for the development of pharmaceutical products through a methodical approach to design of experiments and understanding the design space (variability) of the product. To complement this work, the Xavier team is developing the Supply by Design methodology through the Good Supply Practices to ensure the continued safety and efficacy of product throughout its lifecycle. And finally, industry tends to have concerns about linking quality concepts to business needs, thinking that they are a conflict of interest. However, good quality drives a thriving business, and a successful business is needed in order to continue to serve the patients in need. As a result, the Xavier team has ensured that the solutions are business minded and commensurate with the need.
Table 1: Current State versus Xavier Approach.
Identified Areas For Supply Chain Improvement
A Pareto analysis of the resulting cause and effect scores yielded a rank order of the failure modes in alignment with the critical criteria, and also enabled the team to identify high level themes of failures present in the data — again, related to risks they themselves induce into the supply chain management process. The three main areas for improvement identified were:
- Lack of product and process knowledge and development
- Poor supply chain development and management
- Misaligned and conflicting internal behaviors
The following sections will further explain each area.
- Lack of Product and Process Knowledge and Development
At the outset of this supply chain initiative, the original assumption was that suppliers cannot consistently provide what is needed. However, through our research we discovered that manufacturers often: do not know what specifications are actually needed, do not involve suppliers in development discussions at all or at the right time, do not explore the full expertise of suppliers, do not understand their own process well enough to know how the incoming material will impact their process, and do not ask for the process capability of their suppliers.
- Poor Supply Chain Development And Management
Many manufacturers have supplier selection and supplier approval processes in place, but speed-to-market demands often result in circumvention of these processes. In addition, we found that supply agreements often conflict with the requirements of other agreements, such as the quality agreement and IP protection, and drive the wrong behavior on both sides of the relationship. Additionally, although supplier qualification is not a new concept, we found that the elements of risk assessed often are not representative of key cross-functional requirements and therefore do not provide a complete representation of risk.
- Misaligned And Conflicting Behaviors
Data from manufacturers revealed that, throughout product, process, and supply chain development, a lack of internal involvement from cross-functional representatives consistently leads to a lack of alignment, which results in increased risk. There is no harmonized approach to understanding how to align internal objectives, involving the right groups at the right time, delineating roles and responsibilities, and knowing when and how to engage suppliers. There is a perception that manufacturers cannot share important information with their suppliers, and this lack of transparency hinders suppliers from being able to perform well for the manufacturers. Additionally, manufacturers historically have conducted supplier qualifications to determine the risk levels of their suppliers, but they do not assess the risk they bring to the relationship. Self-assessment would allow manufacturers to avoid false starts, delayed timing, poor product performance, and increased risk to product quality.
Key Aspects Of Good Supply Practices
Based on these areas for improvement, the team is developing the following good supply practices (GSPs):
- Development of pragmatic (i.e., “business smart”) best practices that can be implemented irrespective of company size
- Development of an lifecycle management model for supply chain development and implementation that guides: purpose-driven cross-functional involvement, goals for each stage to maximize alignment, what to measure to determine the success of each stage of development, who the decision makers are, and when to involve suppliers
- Establishment of a Self-Qualification process for manufacturers to to understand and ultimately mitigate risk they induce into the product, process, and supply chain development
- Establishment of a holistic approach to supplier qualification by incorporating risk decisions beyond quality that typically are omitted, such as technological capability, business capability and alignment, operational capability and social responsibility success factors.
- An easy to use tool to identify and clarify what information truly is confidential versus what opportunities there are for transparency, enabling suppliers to better support manufacturers. The tool identifies the benefits and risks of sharing various information so the company can make the best decision for their operations.
- An easy to use tool to identify the role various cross-functional groups/perspectives add to the robustness of the supplier selection and lifecycle management process. The tool includes information on the value each functional group brings to the discussion, as well as the information those functional groups need from the discussion. The organization can then determine which cross-functional team make-up is best for their operations.
- Development of when, how and why to use the cross-functional team guides organizations through how to manage the supply chain lifecycle in a way that is business smart and commensurate with the need.
Looking Ahead
As the Xavier Team is finalizing work on the Good Supply Practices through a methodical Supply by Design process, work is being done to find a global platform in order to increase the access of these solutions to the life science industries. Considering the parallels with Quality by Design principals, the Xavier team would like to have the Good Supply Practices adopted by the International Conference on Harmonization (ICH), and are pursuing possible adoption by the World Health Organization. As our work is concluding, we are running feasibility studies on the solutions and will be running pilot studies in the third quarter of 2017. If you are interested in becoming involved, please contact Marla Phillips at phillipsm4@xavier.edu, or 513-745-3073.
The good supply practices will also be covered in depth at the 2017 FDA/Xavier PharmaLink Conference, held March 14-17. Join us to provide your input and become involved!
About The Author
 Marla A. Phillips, Ph.D. joined Xavier University in 2008 as the director of Xavier Health, where she leads initiatives with FDA officials and pharmaceutical and medical device professionals. Marla began working in the pharmaceutical industry for Merck in 1996, where she took on roles of increasing responsibility, culminating in position of head of quality operations at the Merck North Carolina facility. She holds a B.S. in chemistry from Xavier University, and a Ph.D. in organic chemistry from the University of North Carolina – Chapel Hill.
Marla A. Phillips, Ph.D. joined Xavier University in 2008 as the director of Xavier Health, where she leads initiatives with FDA officials and pharmaceutical and medical device professionals. Marla began working in the pharmaceutical industry for Merck in 1996, where she took on roles of increasing responsibility, culminating in position of head of quality operations at the Merck North Carolina facility. She holds a B.S. in chemistry from Xavier University, and a Ph.D. in organic chemistry from the University of North Carolina – Chapel Hill.