Lessons Learned Help Pfizer Accelerate Biotherapeutic Development
By Rob Wright, Chief Editor, Life Science Leader
Follow Me On Twitter @RfwrightLSL

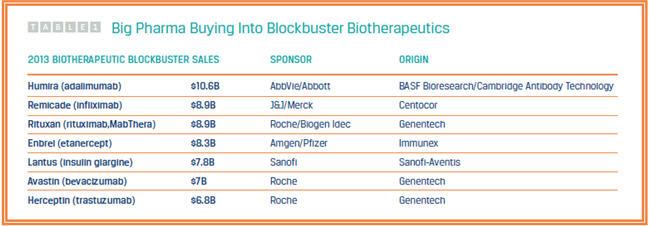
Six of the seven blockbuster biotherapeutics belonging to Big Pharma have roots to small biotechs (see Table 1). So too does Tim Charlebois, Ph.D., VP of technology and innovation strategy within Pfizer’s biotherapeutics pharmaceutical sciences (BTx Pharm Sci). Part of the company’s drug development organization, the 700-person BTx Pharm Sci group operates between Pfizer’s biotherapeutics research and biotech manufacturing organizations. One of the challenges facing Pfizer is an increase in the “perceived” distance between internal operations resulting from the company’s significant growth.
Since 1990 the company has acquired the likes of Warner-Lambert, Pharmacia, and Wyeth and grown from $2.8 billion, to $51.5 billion in 2013. Charlebois’s charge — help Pfizer accelerate its biotherapeutics development program by getting the company’s manufacturing and biotherapeutics research organizations to work together more closely — was much like that at the small biotech where he began his career. According to Charlebois, it involves learning what kinds of new products will be emerging from research taking place upstream, and preparing the organization downstream to develop and manufacture. But it also involves knowing the technology existing downstream and communicating these capabilities upstream, and where Charlebois began the process of decreasing the “perceived” distance between biotherapeutics research and manufacturing by applying lessons learned in small biotech.
The Difference Between Having The Capabilities And Knowing The Capabilities
Charlebois began his career in 1990 at a small company (< 600 employees) — Genetics Institute — a Cambridge, MA-based biotech which had developed a reputation as a technology powerhouse. Like many biotech alumni, his route to Big Pharma came via acquisition, first through Wyeth and then to Pfizer. Charlebois recalls a lesson he learned while at those smaller companies. “I witnessed really close and integrated processes in which biotherapeutics being developed actually matriculated into commercial products within the same site,” he says. Executing end-to-end biologics development is much easier when R&D and manufacturing operate in the same building; people at the beginning stages can see what is going on at the end, are aware of the technology in place, and benefit from being able to have frequent, face-to-face communication.
As you can imagine, when it came to executing end-to-end biologics, it was a different experience for him at a company the size of Pfizer. “When you have very largescale operations, in order to be competent in any particular area, you have to really focus, because the company’s size can be overwhelming,” Charlebois says. For example, just in the United States, Pfizer operates eight geographically dispersed R&D centers. According to Charlebois, the practicality of focusing within one’s own discipline creates a “disconnect” between R&D and manufacturing. “People early on in research can forget that what they’re doing is influencing eight years’ worth of work downstream.” Conversely, Charlebois has seen and read about disconnects occurring between drug designers and commercializers, including a few cases where, despite R&D proof-of-concept metrics being met, the commercial organization did not want to develop the product.
To eliminate disconnects between R&D and manufacturing and across functional boundaries, Charlebois and his five-member “technology and innovation strategy” team work with multidisciplinary initiative teams to align on long-term direction, establish plans, and provide access to funding and management support. Since teams are almost always composed of members from different sites, a variety of communication approaches are leveraged to ensure people stay on the same page. Technology teams meet regularly via teleconference or videoconference, and common interest groups in areas such as bioanalytics and bioconjugation hold regular virtual meetings across the network to share data and ideas. Pfizer intranet-based tools are used to support collaboration among teams and to make strategy and progress visible and accessible across the company. The company has a Web-based infrastructure to support innovation communities across Pfizer. This can be used to post challenges and stimulate virtual discussions among like-minded stakeholders. BTx Pharm Sci also holds an annual tech symposium where scientists and engineers come together to discuss data and strategy through workshops and poster sessions.
Initially set up as part of the integration of Wyeth, Charlebois’ team continued as part of Pfizer’s goal to aggressively leverage and integrate science and technology across the company to accelerate biopharmaceutical development. For example, when Pfizer acquired Wyeth, Charlebois put in place a team charged with inventorying and documenting the capabilities of all of the equipment owned within the newly combined company’s manufacturing and development networks, and then making that comprehensive catalog available to scientists and engineers across Pfizer from their desktops. “The idea was to provide staff with this information so, downstream, they could incorporate these improvements and benefits,” he says.
There is a difference between having the capabilities and knowing the capabilities you have. Charlebois relates the following possible scenario. A biotherapeutic being developed in St. Louis, MO could be clinically manufactured in Andover, MA, which could also be used as a launch facility. But if it’s a large-scale biotherapeutic, the process might have to go to Grange Castle, Pfizer’s 90-acre, one-million-square-foot integrated biotechnology plant in Ireland.
The benefit of knowing what technology exists within the network helps Pfizer scientists plan for the most efficient — and possibly quickest — design. For example, a manufacturing process designed and proven in one facility could face costly delays and put clinical trials or launch supplies at risk due to product quality issues that are caused by subtle changes in the sensitive bioprocessing steps used to product a complex biomolecule.
“We’re actually rethinking what ‘end-toend’ comprises by going all the way back to the research stage and trying to connect it closer to the supply phase,” he says.
Structuring The Interfaces
So how did Pfizer create these teams? Let’s start with the structure. As was mentioned, Pfizer’s development organization essentially resides between research and manufacturing. “We develop the processes and make and deliver the clinical supplies so the products can be tested in the clinic,” Charlebois says. “We develop the technology that will be used in commercial manufacturing if it’s successful.” This probably sounds familiar. Within the development organization, Pfizer created two types of interface teams. The first type interfaces upstream between research and early development.
The second type interfaces downstream between later stages of development and manufacturing. “On the upstream, the interface is much more technical around the modalities and the impact of those on development,” he says. Within this interface team, the focus is on product technologies and modalities, trying to determine what they are going to look like and how will they behave. “In the manufacturing [downstream] interaction, the clinical production technologies tend to be very similar to the commercial ones, except for scale,” says Charlebois. “They’re not six packs of 15,000-liter reactors, but 2,500- liter reactors.” According to Charlebois, the similarity between technologies makes for a much more seamless interface between commercial manufacturing and clinical development engineers to work on the technology. Pfizer built the interface teams to look at where they are now and how they intend to harmonize technology going forward, from development into manufacturing across the drug substance, drug product, and analytical areas. “We basically created an interface group that set objectives together on how to improve processes, how to work together, and what are some key technologies we could use to do so.” Both the upstream (research to early stage development) and downstream (later stage development to manufacturing) BTx Pharm Sci interface teams are managed by Charlebois’ team.
 TIM Charlebois, Ph.D.
TIM Charlebois, Ph.D.
VP Of Technology & Innovation Strategy At Pfizer
By managing both the upstream and downstream interface teams, Charlebois’ group can help to ensure end-to end connectivity, while not requiring everybody across the entire space to have to take an interest in everything.
Charlebois advises prior to creating interface teams that will work between organizations, to first create a governance structure for the team within your own organization, as this is the most local and under your control with regard to the setting and managing of both budgets and high-level objectives. “Then have teams provide proposals that drive toward those high-level objectives.” (For more on how Pfizer creates these objectives, see sidebar — What Objectives Are On Your Horizon?)
For the interface between development and manufacturing, there are governance groups which involve senior leadership members. These groups meet regularly to report progress, give and get direction, and receive feedback. Only two layers of management exist between Charlebois’ team and Pfizer’s executive leadership team (ELT). This illustrates the importance placed on this initiative of striving to operate similar to a smaller biotech. “The top leaders are looking for a big impact from these kinds of initiatives, which pushes us to take a bigger-enterprise perspective,” he attests.
Lessons Learned Thus Far
“When we originally formed the technology and innovation strategy group, we actually had individuals working in my group who focused specifically on bioprocess, analytical, formulation, and delivery but reporting directly to me,” shares Charlebois. “They were working with the respective functional lines. We found this created more distance and less of a sense of technology ownership within each of the functional lines than we desired.” These roles were moved instead into the functional lines, and these leaders built “Tech Committees” responsible for overall coordination within their respective disciplines. Charlebois’ team then works to bring together the technology initiatives into impactful strategies to improve the speed, cost, and quality of biotherapeutics development and manufacturing.
Charlebois reminds you to be patient. “Try and have a sense of urgency on the one hand, but also recognize progress takes time,” he says. “I certainly was very impatient initially, and I learned that with a large organization it takes time for understanding to build and for work that contributes in an impactful way to gain traction and deliver.”
To prevent learning a lesson the hard way, such as interface teams developing or taking on too many projects, put a process in place for reviewing, approving, and funding project proposals. It is essential that some funding, and also scientific and engineering bandwidth, be set aside for innovation. With a large portfolio of product candidates to move forward, there can otherwise be a tendency to focus on short-term deliverables and fail to make the improvements that will serve the enterprise in the long term. In other words, if you expect to move the innovation need, don’t allow it to be relegated to nights and weekends.
Also, allow teams to develop and share ideas within a diverse network, even outside of their area of expertise. For example, Charlebois connected with his counterpart on the pharmaceutical interface side to gain insight into the technologies being used and developed in the areas of continuous, portable, modular, and miniature pharmaceutical manufacturing. “While the technologies aren’t all the same, there are lessons to be learned,” he says. In addition, Charlebois was able to help his counterpart network with some people outside of Pfizer who could help them with what they were working on. “It is important to keep in mind, it isn’t the interface team doing the actual work,” he states. “They simply bring the people together to help coordinate the process and direction of innovation, so that people’s efforts are not fragments but rather are connected to a cohesive strategy.”
Pfizer tracks how much activity each person across the Pfizer development, R&D, and manufacturing enterprise dedicates to each project. This benchmarking data is used to ensure that each employee working on a project is allocating the appropriate amount of time and that they clearly understand their deliverables related to that project. “We are trying to make sure we have enough people giving enough of their time to make the big changes we’re looking for,” he says.
If your goal is to accelerate your biotherapeutics drug development and bring R&D and manufacturing closer together, Charlebois has one final piece of advice — plan well. “We have a large portfolio, so there is a lot of planning to make sure the number of drug development projects being taken on is in alignment with our capacity to execute on their development. The last thing you want is to create a process development improvement project and have it end up as a bottleneck, slowing down a drug’s development.”
What Objectives Are On Your Horizon?
One of the challenges of a company the size of Pfizer is to get employees to think beyond their own day-to-day world and focus on how what they do impacts the company as an enterprise. Tim Charlebois, Ph.D., VP of technology and innovation strategy for biotherapeutics pharmaceutical sciences, BTx Pharm Sci, believes that to overcome this, it is essential to create a culture where people can believe in the value of focusing on the long term. “If you create a culture in which it’s seen as indulgent to think beyond today, then you’re going to get people keeping their heads down and not thinking ahead,” he says. Thus, Pfizer management has been working very hard to communicate a constant and consistent culture of accountability and innovation — referred to as “Own It.” To get people to think long term and more innovatively, Pfizer created a science-based strategy for sustainable innovation with three horizons. Horizon 1 involves the most immediate objective – deliver the portfolio. Horizon 2, intermediate, stands for innovating new capabilities. And Horizon 3 involves creating the R&D ecosystem of the future. Folks within Pfizer began working on the aspirational Horizon 3 objectives first, which were five to 10 years into the future. From there, they worked backward. In taking this approach, the company created Horizon 2 (intermediate) objectives, geared toward achieving Horizon 3, and immediate objectives, geared toward achieving Horizon 2. “We’ve created teams to help guide the expertbased prosecution of ideas and then collect those into bigger buckets that can drive toward those high-level goals,” Charlebois states.
