Industry 4.0: Improving Performance Of Pharma Manufacturing & Aseptic Processing
By Richard Levy, Ph.D., PDA

Aseptic processing has been an interest of mine since I first joined Millipore Corporation as a microbiologist in the late 1980s and began to focus on sterilizing filtration. Despite the challenges of maintaining an aseptic environment and producing a sterile drug product, I believed that someday the science and technology would allow manufacturers to have the same level of confidence in aseptically processed products as they had in terminally sterilized products.
In 1994, Michael Korczynski published a paper in the PDA Journal of Pharmaceutical Science and Technology titled, “The Integration of Process Analytical Technologies, Concurrent Validation, and Parametric Release Programs in Aseptic Processing.”1 I was energized by his vision of a future when the integration of process analytical technology (PAT), concurrent validation, and parametric release programs could be used to improve product and process quality during manufacturing and, as he summarized, result in economic and efficiency improvements. In the paper, Korczynski wrote about the innovative use of online or in-line assay methods and measurement techniques, using a hypothetical example. And he described the value of utilizing in-process, real-time data. While much more has been published on the subject by many authorities area since then, Korczynski’s article is worth reading even today.
Fast forward to 2014. Since that PDA Journal article sparked my interest in this topic, I have attended numerous meetings on pharmaceutical and biopharmaceutical manufacturing. At those meetings, I was always struck by how frequently we talked about how to move toward Korczynski’s vision yet lamented how little was changing with aseptic processing (and pharma manufacturing in general). The reality, however, was that definite improvements were underway. Around that time, I read an article on how Merck & Co. had applied information technology tools to optimize yields in vaccine production.2 My interest piqued, I contacted Michele D'Alessandro , VP and CIO–manufacturing information technology at Merck, and invited her to present a paper on the project at the 2015 PDA/FDA Vaccines Conference. Michele accepted, and her presentation, titled “Case Study: Using Information Technology-Based Tools to Optimize Yields in Vaccine Production,” demonstrated how actionable insights to improve yield could be applied at every manufacturing step, from fermentation to purification.3 She also highlighted how a comprehensive understanding of the application of Big Data technologies could result in deep insights into manufacturing processes. The pieces of the data puzzle seemed to be falling into place.
Fast forward again to 2016. I heard a very interesting presentation by Andreas Traube of Fraunhofer IPA at the PDA Europe Annual Meeting titled “Industrie 4.0 — Readiness for Pharmaceutical Companies.”4 Andreas said that we were now on the edge of the fourth Industrial Revolution, driven by connectivity and holistic integration, which will enable new manufacturing ecosystems that connect the factory with the production network, as well as the consumer through new design of infrastructure and optimized networks. Traube’s thesis was that there is business potential for pharmaceutical manufacturing to capitalize on this revolution. This was indeed exciting. I have revisited those slides over and over since then. However, I had missed one interesting potential benefit, which I am sure was there, and it is possibly the best end to the story of enhancing aseptic processing.
Fast forward to today. I just read that Google is rolling out new features to its Google Flight search engine, which launched in 2011.5 As a frequent traveler, I am always trying to get the upper hand on the weather, the airlines, and flight delays. Using Google Flight and other smartphone apps that tap into multiple flight databases, I usually learn when my flights have been delayed or cancelled just before I receive the dreaded “this is Southwest Airlines calling with a flight status update” call. One of my apps reports flight status changes in what appears to be real time. For example, it is not uncommon for me to learn ahead of time when Southwest (I do love the airline) has changed a plane’s arrival gate to the one where I was supposed to depart, signaling a potential delay for my flight. Using this advance knowledge, I can adjust my travel plans as necessary, in most cases leading to a better outcome. Google hopes it will be able to predict some delays well in advance of official confirmation by the airline, and it has algorithms that it uses to make those calls much earlier than I can expect to hear about them now, even using my apps. The program will also provide reasons for the delays, which is also useful to me, especially if I’m trying to decide whether to switch to another airline or return home and try again tomorrow.
Having said all this, the technology is not perfect, and I have been fooled. However, my positive experiences makes me wonder if drug companies soon will be able do the same sort of thing as Google Flights— that is, predict issues we are likely to experience in pharmaceutical manufacturing and aseptic processing.
At the latest meeting of the PDA Manufacturing Science and Operations (MSOP) Steering Committee (of which Michele D’Alessandro is a member), we talked about an ongoing project on manufacturing intelligence, which is looking at the application of Big Data. We also discussed a project on real-time release of drug products, including those aseptically processed. And, industry experts spoke to us on continuous manufacturing and artificial intelligence, as it will undoubtedly be applied to pharmaceutical and biotech manufacturing.
Considering the forward thinking of the MSOP Steering Committee and industry thought leaders, as well as the rapid advancement of technology in general (e.g., Google Flights), the next generation of pharmaceutical manufacturing will undoubtedly be driven by Industry 4.0, Big Data, augmented reality, and artificial Intelligence.
Looking at the aseptic manufacturing suite today, we can see where the diversity of data will come from, where it will go, and how it will return. Figure 1 supports our vision of the future in aseptic processing. Specifically, it identifies several areas that may already benefit from some degree of Industry 4.0 connectivity: sterile manufacturing, manufacturing support, laboratory, cloud, and patient.
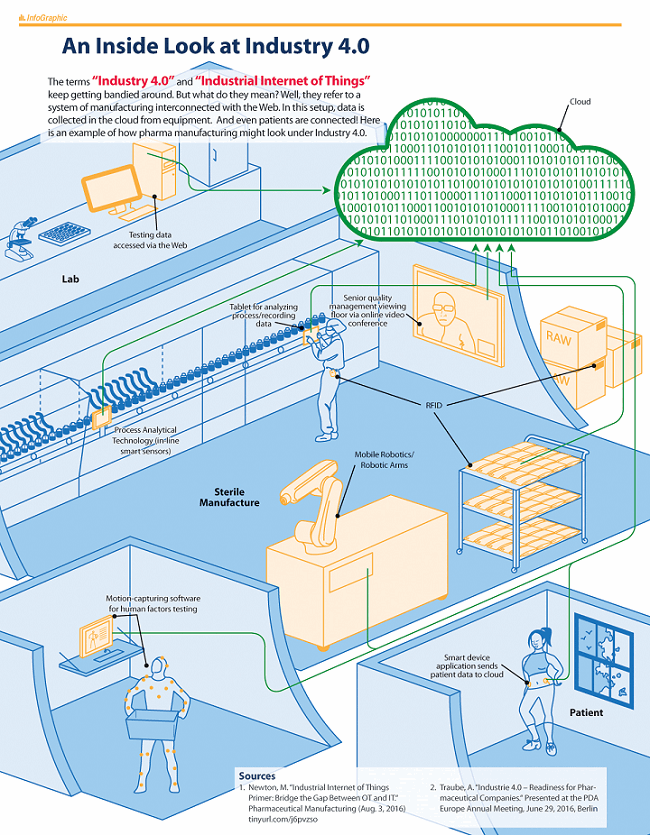
Figure 1: An industry look at Industry 4.0 and the industrial internet of things related to aseptic processing (developed by PDA staff). Courtesy of the March 2017 PDA Letter.
In sterile manufacturing, PAT using in-line smart sensors on the manufacturing line would be linked to the shop floor, to the lab, and to the cloud. Operators would use tablets to review standard operating procedures (SOPs), analyze real-time data, and input observations. Management and quality assurance staff could communicate with the shop floor via online video conferencing. Mobile robotics and RFID information about both product and packaging would be fed to the cloud, and the cloud would send feedback in real time.
Manufacturing support would incorporate connected planning and control systems. Connected supply chain and patient information could drive manufacturing and distribution campaigns. The human-machine connection could capitalize on decentralized real-time process information and provide online training and education. Connections between support staff and machines could drive technical modification, maintenance, and repairs. And connection to quality and regulatory information could drive process improvements within manufacturing windows.
Laboratory systems would receive information from connected online sensors, and feedback and historical information from the cloud.
Decentralized real-time collection processes would be routed to the cloud, where Big Data tools, data storage, and AI would team up to analyze the data and provide feedback to the shop floor. And that feedback from the cloud could be predictive information that reaches machines and operators in real time.
Patients could provide more feedback on human factors, while connected health systems supply health outcomes and pharmacovigilance data.
I predict it will not take another 30 years before we capitalize on the original concepts Michael Korczynski envisioned in 1994. With the forward thinking of the PDA MSOP Steering Committee and our industry leaders — and the convergence of Industry 4.0, PAT, Big Data, augmented reality, and artificial intelligence — we should soon be capable of integrating the predictive resources that will enable us to achieve the highest levels of sterility assurance with aseptic processing.
What are you and your company doing to embrace the future? Please let me know in the Comments section below.
References:
- Korczynski, M (2004). The integration of process analytical technologies, concurrent validation, and parametric release programs in aseptic processing. PDA J Pharm Sci and Tech, 58, 181-191.
- Henschen, D (2014). Merck optimizes manufacturing with big data. InformationWeek. https://www.informationweek.com/strategic-cio/executive-insights-and-innovation/merck-optimizes-manufacturing-with-big-data-analytics/d/d-id/1127901 .
- D'Alessandro, M (2015). Case study: Using information technology-based tools to optimize yields in vaccine production. [Presentation]. Presented at the 2015 PDA/FDA Vaccines Conference, Bethesda, Md.
- Traube, A (2016). Industrie 4.0 – readiness for pharmaceutical companies. [Presentation]. Presented at 2016 PDA Europe Annual Meeting. Berlin, Germany.
- Perez, S (2018). Google Flights will now predict airline delays – before the airlines do. TechCrunch. https://techcrunch.com/2018/01/31/google-flights-will-now-predict-airline-delays-before-the-airlines-do/.
About The Author:
 Richard Levy, Ph.D., is currently senior VP of scientific and regulatory affairs at the Parenteral Drug Association (PDA). In this capacity, he is responsible for directing and managing the scientific, regulatory, and quality activities of a 10,000-member association focused on pharmaceutical and biotechnological manufacturing. He is also responsible for two of the association’s publications: the PDA Journal of Pharmaceutical Science and Technology and the PDA Letter.
Richard Levy, Ph.D., is currently senior VP of scientific and regulatory affairs at the Parenteral Drug Association (PDA). In this capacity, he is responsible for directing and managing the scientific, regulatory, and quality activities of a 10,000-member association focused on pharmaceutical and biotechnological manufacturing. He is also responsible for two of the association’s publications: the PDA Journal of Pharmaceutical Science and Technology and the PDA Letter.
