How To Prevent Sample Contamination Of ICP-MS Systems In Pharmaceutical Processing
By Robert J. Thomas, Scientific Solutions

The application landscape of inductively coupled plasma mass spectrometry (ICP-MS) has changed considerably since the technique was first commercialized in 1983. In the early years, it was mainly the environmental and geological communities that realized its benefits. Then, as its capabilities became better known and its performance improved, other application areas like clinical, toxicological, and semiconductor markets embraced the technique. Today, ICP-MS, with all its performance and productivity enhancement tools, is the most dominant trace element technique, and it is being utilized in other exciting areas, including nanoparticle research, oil exploration, and pharmaceutical manufacturing.
ICP-MS’ rapid growth for pharmaceutical testing has been driven by recent regulations described in United States Pharmacopeia (USP) General Chapters <232> and <233>1 and International Conference on Harmonization (ICH) Guidelines2 that require the industry to monitor 24 elemental impurities in drug compounds and raw materials using either ICP-OES (inductively coupled plasma optical emission spectrometry) or ICP-MS. However, it’s important to emphasize that manufacturing facilities of pharmaceutical companies have never really been required to use plasma spectrochemical-based techniques before. ICP-MS, in particular, has been considered more applicable to the demands of the drug development and discovery process. For that reason, it has primarily been used by R&D groups that have analytical chemists with a high level of expertise. With the approval of these USP methods and ICH guidelines, pharmaceutical production facilities will probably have to invest in this new technology and hire technicians who have very little experience of using sophisticated analytical instrumentation to operate this equipment. This article emphasizes the importance of implementing a regular routine maintenance schedule, so problem areas associated with instrumental components that are most susceptible to sample blockage, drift, and signal instability can be avoided.3,4
Benefit Of Routine Maintenance In ICP-MS
The fundamental principle of ICP-MS, which gives the technique its unequalled isotopic selectivity and sensitivity, also unfortunately contributes to some of its weaknesses. The fact that the sample “flows into” the spectrometer and is not “passed by it” at right angles, such as flame atomic absorption (AA) and radial ICP-OES, means that the potential for thermal problems, corrosion, chemical attack, blockage, matrix deposits, and drift is much higher than with the other atomic spectrometry (AS) techniques. However, being fully aware of this fact and carrying out regular inspection of instrumental components can reduce and sometimes eliminate many of these potential problem areas. There is no question that a laboratory that implements a routine maintenance schedule stands a much better chance of having an instrument ready and available for analysis whenever it is needed, compared to a laboratory that does not give importance to these issues and assumes the instrument will look after itself.
The areas of the instrument that require inspection and maintenance on a routine basis include the following components:
- Sample introduction system
- Plasma torch
- Interface region
- Ion optics
The majority of imprecision problems in ICP-MS arise due to the potential for sample matrix to deposit itself on the nebulizer tips, the sample injector of the torch, the interface cones, and/or the ion optics. In this article, we will focus on the interface cones and the ion optics region, which are the root of most of the drift and instability problems experienced in ICP-MS.
Interface Region
As the name suggests, the interface is the region of the ICP mass spectrometer where the plasma discharge at atmospheric pressure is “coupled” to the mass spectrometer at 10−6 torr by way of two interface cones — a sampler and skimmer. This coupling of a high-temperature ionization source such as an ICP to the metallic interface of the mass spectrometer imposes demands on this region of the instrument that are unique to this AS technique. When this is combined with matrix, solvent, and analyte ions together with particulates and neutral species being directed at high velocity at the interface cones, it results in an extremely harsh environment. The most common types of problems associated with the interface are blocking or corrosion of the sampler cone and, to a lesser extent, the skimmer cone. A schematic of the interface cones showing potential areas of blockage and/or corrosion is shown in Figure 1.
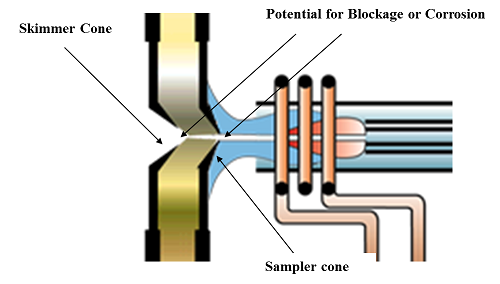
Figure 1: The interface cones showing potential areas of blockage or corrosion
A blockage or corrosion is not always obvious, because often the buildup of material on the cone or corrosion around the orifice can take a long time to reveal itself. For that reason, the sampler and skimmer interface cones have to be inspected and cleaned on a regular basis. The frequency will often depend on the types of samples being analyzed and on the design of the ICP mass spectrometer. For example, it is well documented that a secondary discharge at the interface can prematurely discolor and degrade the sampler cone, especially when complex matrices are being analyzed or if the instrument is being used for high sample throughput.
Besides the cones, the metal interface housing itself is also exposed to the high-temperature plasma. Therefore, it needs to be cooled by a recirculating water system, usually containing some kind of antifreeze or corrosion inhibitor or by a continuous supply of mains water. Recirculating systems are probably more widely used because the temperature of the interface can be controlled much better. There is no real routine maintenance involved with the interface housing, except maybe to check the quality of the coolant from time to time, to make sure there is no corrosion of the interface cooling system. If for any reason the interface gets too hot, there are usually built-in safety interlocks that will turn the plasma off. Some useful hints to prolong the lifetime of the interface and cones include the following:
- Check that both sampler and skimmer cones are clean and free of sample deposits. The typical frequency is weekly, but that will depend on sample type and workload.
- If necessary, remove and clean cones using the manufacturer’s recommendations. Typical approaches include immersion in a beaker of weak acid or detergent placed in a hot water or ultrasonic bath. Abrasion with fine wire wool or a coarse polishing compound has also been used.
- Never stick any wire into the orifice; it could do permanent damage.
- Nickel cones will degrade rapidly with harsh sample matrices. Use platinum cones for highly corrosive solutions and organic solvents.
- Periodically check cone orifice diameter and shape with a magnifying glass (10x to 20× magnification). An irregular-shaped orifice will affect instrument performance.
- Thoroughly dry cones before installing them back in the instrument because water/solvent could be pulled back into the mass spectrometer.
- Check coolant in recirculating system for signs of interface corrosion such as copper or aluminum salts (or predominant metal of interface).
Ion Optics
The ion optic system is usually positioned just behind or close to the skimmer cone to take advantage of the maximum number of ions entering the mass spectrometer. There are many different commercial designs and layouts, but they all have one attribute in common, and that is to transport the maximum number of analyte ions while allowing the minimum number of matrix ions through to the mass analyzer.
The ion-focusing system is not traditionally thought of as a component that needs frequent inspection, but because of its proximity to the interface region, it can accumulate minute particulates and neutral species that over time can dislodge, find their way into the mass analyzer, and affect instrument performance. Signs of a dirty or contaminated ion optic system are poor stability or a need to gradually increase lens voltages over time. For that reason, no matter what design of ion optics is used; inspection and cleaning every three to six months (depending on workload and sample type) should be an integral part of a preventive maintenance plan. Some useful maintenance tips for the ion optics to ensure maximum ion transmission and good stability include the following:
- Look for sensitivity loss over time, especially in complex matrices.
- If sensitivity is still low after cleaning the sample introduction system, torch, and interface cones, it could indicate that the ion lens system is becoming dirty.
- Try retuning or reoptimizing the lens voltages.
- If voltages are significantly different (usually higher than previous settings), it probably means lens components are getting dirty.
- When the lens voltages become unacceptably high, the ion lens system will probably need replacing or cleaning. Use recommended procedures outlined in the operator’s manual.
- Depending on the design of the ion optics, some single-lens systems are considered consumables and are discarded after a period of time, whereas multicomponent lens systems are usually cleaned using abrasive papers or polishing compounds and rinsed with water and an organic solvent.
- If cleaning ion optics, make sure they are thoroughly dry because water or solvent could be sucked back into the mass spectrometer.
- Gloves are usually recommended when reinstalling an ion optic system because of the possibility of contamination.
- Do not forget to inspect or replace O-rings or seals when replacing ion optics.
- Depending on instrument workload, you should expect to see some deterioration in the performance of the ion lens system after three to four months of use. This is a good approximation of when it should be inspected and cleaned or replaced if necessary.
- With some instruments, you might need to break the vacuum to get to the ion optic region. Even though vacuum can be reestablished very quickly, this should be a consideration when carrying out your own ion lens cleaning procedures.
Summary
This article highlights the importance of keeping the ICP-MS interface and ion optic region free from sample contamination. By carrying out some basic troubleshooting routines and implementing a regular routine maintenance schedule, typical problematic areas associated with pharmaceutical matrices can be avoided.
For more detailed information about maintaining the other instrumental components, please refer to the chapter on routine maintenance in the book Measuring Elemental Impurities in Pharmaceuticals4, on which this article was based.
References:
- Elemental Impurities in Pharmaceuticals: Updates: USP Website: http://www.usp.org/chemical-medicines/elemental-impurities-updates
- International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use http://www.ich.org/products/guidelines/quality/article/quality-guidelines.html
- "Approaches to Maximize Performance and Reduce the Frequency of Routine Maintenance in ICP-MS," R. Brennan, J. Dulude, R.J Thomas, Spectroscopy, 30(10), 12-25, 2015
- Measuring Elemental Impurities in Pharmaceuticals: A Practical Guide, Robert. J. Thomas; CRC Press, Boca Raton, FL, ISBN 9781138197961, February, 2018
About The Author:
 Robert J. Thomas has worked in the field of trace element analysis for over 40 years, including 22 years for an atomic spectroscopy instrumentation manufacturer and 15 years as a principal of his own consulting company. He has authored over 80 publications on trace element analysis including three textbooks on ICP-MS. His latest book is entitled Measuring Elemental Impurities in Pharmaceuticals: A Practical Guide. He is currently editor and frequent contributor to the Atomic Perspectives column in Spectroscopy magazine. He has an advanced degree in analytical chemistry from the University of Wales in the UK, and is a Fellow of the Royal Society of Chemistry (FRSC) and a Chartered Chemist (CChem).
Robert J. Thomas has worked in the field of trace element analysis for over 40 years, including 22 years for an atomic spectroscopy instrumentation manufacturer and 15 years as a principal of his own consulting company. He has authored over 80 publications on trace element analysis including three textbooks on ICP-MS. His latest book is entitled Measuring Elemental Impurities in Pharmaceuticals: A Practical Guide. He is currently editor and frequent contributor to the Atomic Perspectives column in Spectroscopy magazine. He has an advanced degree in analytical chemistry from the University of Wales in the UK, and is a Fellow of the Royal Society of Chemistry (FRSC) and a Chartered Chemist (CChem).
