Comparing Continuous And Batch Processing In Downstream Purification

Current state-of-the-art continuous manufacturing technologies are being developed and implemented to manufacture a wide variety of products including monoclonal antibodies, recombinant proteins, and other biological modalities. Though upstream fed batch and perfusion bioreactors unit processes are relatively mature, downstream process unit operations are less mature. In this case study, Catalent compared the productivity of purifications running in batch versus continuous mode. Both Protein A capture resins evaluated demonstrated increased productivity with lower buffer volume in continuous mode compared to batch mode.
The Challenge
Several vendors offer development-scale to commercial-scale continuous downstream chromatography systems that support capture, intermediate, and polishing purification steps. When adopting continuous technology, it is critical to demonstrate that continuous downstream processes are comparable to batch-mode downstream processes as well as providing the supporting comparability data for technical and business decisions and regulatory submission documentation.
The Catalent Solution
In this study, the productivity of purifications using two different Protein A capture resins running in the batch mode (i.e., one large column) were compared to purifications running in the continuous mode (i.e., multiple columns). The wash, equilibration, and separation buffer were the same and the fluid flows were appropriate for the column size. For the two resins systems, the continuous process was up to three times more productive than the batch process to produce the same amount and quality of protein (Tables 1 and 2).
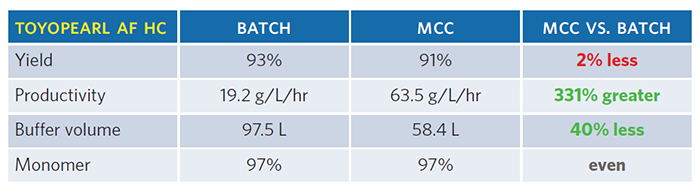
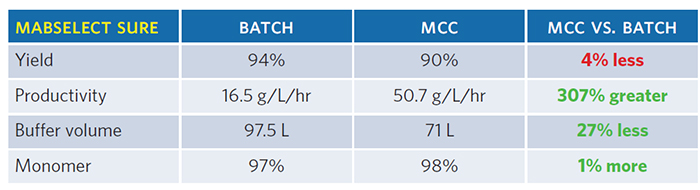
Conclusion
Both Protein A capture resins demonstrated increased productivity with lower buffer volume in continuous mode versus batch mode. At Catalent, we implemented a few unit operations in a continuous mode, enabling reduced risk and development time to move a biologic molecule to the clinic.
