An Evaluation Of A Closed Sterile Transfer Process For Aseptic Filling
By Tara Scherder, James Agalloco, David Hussong, and Leonard Mestrandrea
Critical control points for conventional aseptic processing are most often related to contamination resulting from human intervention5 and exposure of critical surfaces before and during fill. Extensive resources are required to install and maintain controls for these typical failure modes which can lead to two critical issues: shortages of essential medicines and inability to meet the timelines critical to control emerging pandemic threats.1,2,3,4,5,6,7
These failure modes are virtually eliminated for closed processing using closed sterile transfer technology. This entirely different paradigm warrants an original approach to manufacturing process control and assurance of sterility. We present a totality of evidence approach to show superior sterility performance of a well-designed closed system compared to conventional aseptic processing, and to design the control strategy.
Process Description
The peer-reviewed evidence summarized in this review was collected from experiments by MedInstill Development LLC (New Milford, CT) using their INTACT filling technology and the associated protocols. A closed system filling technology8 is an automated sterile connector technology by which pre-sterilized closed containers are filled through an engineered and controlled passage enabling the filled product, the internal container, and the closure system critical surfaces to avoid exposure to the background environment. When using this manufacturing technology, the sterile solution remains within a sterile fluid path at all times, and environmental exposure — including direct human intervention with critical (sterile) surfaces — is eliminated. Figure 1 illustrates the sequence of a closed system filling process.
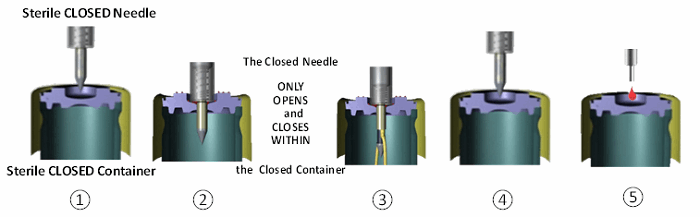
Figure 1 – Closed system filling process sequence: (1) closed container and closed needle ready to fill (both radiation sterilized), (2) container penetrated by closed needle, (3) needle opens inside container, dispenses liquid, closes inside container, (4) closed needle exits container, container opening recloses, closed needle ready for next container, and (5) closed container externally resealed
Risk Comparison And Evidence Approach
The performance of open aseptic processing is inherently variable and imperfect, yet its acceptability is supported in part by the conduct of thousands of media fill tests performed worldwide. Many years of successful open aseptic processing have provided a plethora of product and media challenge evidence that risk of contamination can be adequately managed through design of processing equipment, stringent environmental controls, personnel training, and equipment — and confirmed by ongoing monitoring.9,10,11,12
Performance data for any new process is limited in comparison to the legacy aseptic process. However, this lack of specific empirical data does not necessarily infer that there is more patient/consumer risk. In this case, a totality of evidence approach can be used to compare the closed system technology to conventional aseptic processing. That is, all relevant evidence can be holistically evaluated to make a non-inferiority or superiority claim.
This evaluation requires a comparison of risk of the two process technologies from human intervention and environmental deposition. Details for this comparison are divided into subsections below including: general discussion of the failure modes, technology features that prevent microbial contamination, performance evidence, and the control cascade strategy to maintain sterility assurance at levels superior to conventional aseptic processing.
Comparison Of Failure Mode Through Human Intervention
During conventional aseptic processing, both microbial contaminants and nonviable particulates can settle on/enter open containers, closures, and exposed product contact parts. Probability of contamination increases with human intervention and is minimized by application of quality risk management (QRM) principles. This mitigation process is subject to uncertainty, as it depends on accurate identification and assessment of risk and a myriad of operational controls, including compliance with standard operating procedures designed to prevent contamination.13,14
In contrast, in a closed system filling process, product contact surfaces are not exposed at any time before, during, or after the filling process. Additionally, all components including filling needles are assembled and closed prior to sterilization. Thus, contamination resulting from exposure to human intervention is eliminated by design.9
Comparison Of Failure Mode Through Environmental Deposition
The total accumulation of bioburden on the critical surfaces is a function of three factors: surface area exposed, duration of exposure, and of number of microorganisms present in the environment.15 The closed sterile transfer system operates in a controlled non-classified (CNC) environment.16,17 There is negligible impact of the three bioburden accumulation factors for the product contact surfaces of this closed system,18 as opposed to the conventional aseptic processing in an ISO 5 environment9 based on the following:
- The total amount of deposition per unit of the product contact surfaces for a closed system is negligible compared to a typical open aseptic process. (A comprehensive comparison of deposition demonstrating superior performance of the closed system will be presented in a future publication.)
- In the case of contamination deposition from environment on the fill point, the technologies prevent any deposited bioburden from being introduced into the container.
Thus, contamination from the processing and background environments, worsened through human intervention, is virtually eliminated. A controlled environment for closed processing is not necessary but can be incorporated as added safety against deposition of both viable and nonviable particles.
Prevention Of Contamination By Surface Bioburden
In a closed system, the ingress of contamination can only occur by transfer into the closed containers from surface deposition onto the closed needle and/or septum. Virtual elimination of this risk can be achieved through a combination of technologies, including:
- All components, including filling needles, are assembled and closed prior to sterilization.
- Irradiation of all disposable components
- The use of closed sterile transfer connectors for all connections
- Engineering of physical dimensions and material of construction of the outer needle and container closure (septum)
- A septum material that is self-closing upon needle withdrawal and then physically sealed
- Product dependent and redundant sterile filtration prior to filling
- Specific operating procedures/design elements for filling setup and operation:
- No manual intervention required for initial setup of the filling needles
- Replacement of disposable filling kits in contact with container opening/closure following catastrophic processing conditions without manual intervention
- Design of the filling machine to operate within a CNC environment as defined by ISPE,13,14 including an added total particle sensor for routine enumeration
- A second set of HEPA filters is incorporated over the filling/sealing area including an added total particle sensor for routine enumeration
- As an extreme safety precaution, irradiation (e.g., ultraviolet pulsed light at 254 nm wavelength) of the target surface just prior to piercing and filling can be applied.
Evidence For The Elimination Of Contamination Risk
Precise engineering of the physical dimensions and materials of construction of both the outer needle and septum resulted in effective wiping of contaminants that were deliberately deposited on the septum, effectively preventing contaminants from entering the sterile container. This virtual elimination of contamination risk was evidenced by extensive testing analogous to the biological indicator challenges of a terminal sterilization.
Testing was performed across a wide spectrum of microorganisms and extreme levels of contamination. In addition to the obvious superiority afforded by testing at extreme conditions, this testing design is not subject to the statistical uncertainty inherent in environmental monitoring, media fills, and sterility tests as accepted for conventional aseptic processing.
Success was evident from the absence of growth from media fill experiments following extreme microbiological challenges including:19
- while submerged in microbial suspension of 103 CFU/mL
- after direct application of 106 CFU to the septum (0.01 mL x 108 CFU/mL) before needle penetration and without UV sanitization
- in aerosols containing 104 CFU/m3 (extrapolates to an environment of >3.5 x 108 particles (0.5 µm) per m3, i.e., 100-fold greater viable contamination than total particles permitted in a ISO 8 space)
- after the needle was used to pierce septa 30,000 times
Additional evidence included: (1) the location of contaminants found in scanning electron microscopy of surfaces following piercing and withdrawal of the needle while external needle surfaces and septum were coated with microbiological loads of ≈ 109 CFU/mL, and (2) the absence of microorganisms, including endospores, after ultraviolet pulse light irradiation (254 nm) to the piercing surface just before filling.20
Operation at the extreme conditions described above would certainly lead to failure in conventional processing. This substantial and thorough evidence of contamination prevention establishes the superior performance of the closed system compared to conventional open aseptic processing (irrespective of manual or robotic operations).

Plan for continuous improvement in Aseptic Fill process. Learn the tips to convert your existing system from industry expert Herman Bozenhardt in the webinar:
Critical Control Points/Control Strategy
Substantial evidence has shown that precise engineering of the outer needle and septum to wipe the outer needle and prevent contaminant from entering the sterile container can virtually eliminate the hypothetical risk of contamination during closed processing resulting from environmental deposition. The specific dimensions and material of construction of the container septum and the needle are critical to wiping effectiveness, and are thus critical control points to the process.
The control strategy must ensure consistency of these components, beginning with the molding process. In addition to standard quality testing, vendor relationships must be established that will enable identification of any manufacturing change that could affect material consistency and thus component performance. Container closure integrity and extreme tests like those described above should be performed whenever a process change has been made (at either a vendor or filling site) that could affect individual and/or pairing material consistency, and at some routine frequency designed to prevent potentially contaminated product from release.
Conclusion
Conventional aseptic processing and closed system/closed sterile transfer differ not only in design and operation, but also in the methods to demonstrate assurance of sterility. Conventional aseptic filling requires exquisite controls to protect critical filling materials, and validation relies on media fill methods. In contrast, closed system processing using the closed sterile transfer technology eliminates many of the contamination vectors associated with open aseptic filling, and uses extreme environmental challenges to validate the process.
These fundamental disparities warrant different approaches to assessment and management of contamination risk. A robust comparison of the two processes that considers the totality of evidence can be made.
When closed system filling is performed with the controls described herein, a margin of safety and operating conditions impossible with open aseptic processing can be achieved. This performance difference is clearly established by biological challenge studies that demonstrate microbial exclusion in extreme environmental and surface conditions that no open aseptic process could withstand. This evidence is far more robust than conventional media fill studies performed in a pristine environment. This holistic evaluation leaves no doubt that the closed process can provide superior sterility performance compared to conventional aseptic processing.
Acknowledgements:
The technical assistance of Debashis Sahoo and F. Andreas Toba of Medinstill Development, LLC, is deeply appreciated.
References:
- Gupta, S. 2017. The big one is coming, and it's going to be a flu pandemic. CNN: http://www.cnn.com/2017/04/07/health/flu-pandemic-sanjay-gupta/index.html
- Gates, B. 2017. A new kind of terrorism could wipe out 30 million people in less than a year and we are not prepared. Business Insider: http://www.businessinsider.com/bill-gates-op-ed-bio-terrorism-epidemic-world-threat-2017-2
- Merkel, A. 2015. Joining forces to tackle epidemics. World Health Assembly (G-7): https://www.g7germany.de/Content/EN/Reiseberichte/2015/2015-05-18-bkin-weltgesundheitsversammlung-genf_en.html?nn=1282258
- Gates, B. 2015. The Next Epidemic – Lessons from Ebola. The New England Journal of Medicine: http://www.nejm.org/doi/full/10.1056/NEJMp1502918#t=article
- Brockmann, D. 2014. Understanding and predicting the global spread of emergent infectious diseases. Public Health Forum: http://www.sciencedirect.com/science/article/pii/S0944558714000365
- Stöhr, K. and A. Costa. 2011. Influenza Global Vaccine Supply - Current status of vaccine production and Pandemic preparedness. WHO Global Influenza Programme – Epidemic and Pandemic Alert and Response: http://www.paho.org/hq/index.php?option=com_docman&task=doc_download&Itemid=270&gid=15626&lang=en
- Augustine, J. J. 2017. Drug Shortages and Rapid Price Escalation: The Latest on the Crisis. Journal of Emergency Medical Services: http://www.jems.com/articles/2017/07/drug-shortages-and-rapid-price-escalation.html
- Agalloco, J., J.L. Quick, L. Mestrandrea, and D. Hussong. 2015. Closed System Filling Technology: A New Paradigm. PDA Letter (10): 26 – 28.
- Friedman, R. L. 2005. Aseptic Processing Contamination Case Studies and the Pharmaceutical Quality System. PDA J Pharm Sci Technol. 59(2): 118-126.
- Jones, D. J., P. Topping, and J. Sharp. 1995. Environmental Microbial Challenges to an Aseptic Blow-Fill-Seal Process. PDA J Pharm Sci. Technol. 49(5): 226-234.
- Sinclair, C. S., and A. Tallentire. 1992. Performance of Blow/Fill/Seal Equipment Under Controlled Airborne Microbial Challenges. PDA J Pharm Sci&Tech. 49(6): 294-299.
- Reed, C H., and B. Verjans. 2012. Assessing Filling Technologies for Contamination Risk. BioPharm International 25(3): http://www.biopharminternational.com/assessing-filling-technologies-contamination-risk
- Baseman, H., M. Hardiman, W. Henkels and M. Long. 2016. A Line of Sight Approach for Assessing Aseptic Processing Risk, Part I. PDA Letter 52(6): 22-29; Part II. PDA Letter 52(7): 42-45; Part III. PDA Letter: https://www.pda.org/pda-letter-portal/archives/full-article/a-line-of-sight-approach-for-assessing-aseptic-processing-risk-part-iii
- Akers, J., and J. Agalloco. 2006. The Simplified Akers–Agalloco Method for Aseptic Processing Risk Analysis. Pharma Technol. July 2006.
- Whyte, W. 1986. Sterility Assurance and Models for Assessing Airborne Bacterial Contamination. J Parenteral Sci Technol. 40(5):188-197.
- ISPE. 2011. Sterile Product Manufacturing Facilities, in ISPE Baseline Pharmaceutical Engineering Guide, Vol 6, 2nd edition, International Society for Pharmaceutical Engineering. Tampa, FL.
- ISPE. 2013. Biopharmaceutical Manufacturing Facilities, in ISPE Baseline Pharmaceutical Engineering Guide, Vol 3, 2nd edition, International Society for Pharmaceutical Engineering. Tampa, FL.
- Probst, S., S. Chalk, M. Zicaro, P. McDuff, L. Pranzo, L. Dooley, R. Moser, F. Urbanski, P. Smock and K. Green. 2013. New Challenges to the Cleanroom Paradigm for Multi-Product Facilities. BioPharm International 26(5) http://www.biopharminternational.com/new-challenges-cleanroom-paradigm-multi-product-facilities
- Toba, F.A. Closed System Transfer Technology with INTACT(TM) – A Case Study. ISPE/PQRI/FDA Quality Manufacturing Conference. Emerging Technologies - Part 2. June, 2016. Bethesda, MD.
- Gates, F. L. 1929. A study of the bactericidal action of ultraviolet light. J Gen Physiol. 13(2):231-260.
About The Authors:
 Tara Scherder has over 30 years of experience in the chemical and pharmaceutical industries as a statistician, process engineer, and master black belt. She has functioned as both an in-house and external statistical consultant to drug substance and drug product teams across the product lifecycle for the spectrum of product platforms. As partner at SynoloStats, she passionately shares the opportunity for patient and business benefit through the combination of statistics and process. Tara earned a BS degree in chemical engineering from the University of Pittsburgh and an MS degree in statistics from Carnegie Mellon University. She is an active member of the International Society for Pharmaceutical Engineering (ISPE) and frequently speaks at industry forums on the practical incorporation of statistical methods for lifecycle process validation.
Tara Scherder has over 30 years of experience in the chemical and pharmaceutical industries as a statistician, process engineer, and master black belt. She has functioned as both an in-house and external statistical consultant to drug substance and drug product teams across the product lifecycle for the spectrum of product platforms. As partner at SynoloStats, she passionately shares the opportunity for patient and business benefit through the combination of statistics and process. Tara earned a BS degree in chemical engineering from the University of Pittsburgh and an MS degree in statistics from Carnegie Mellon University. She is an active member of the International Society for Pharmaceutical Engineering (ISPE) and frequently speaks at industry forums on the practical incorporation of statistical methods for lifecycle process validation.
 James Agalloco is president of Agalloco & Associates, a technical service firm to the pharmaceutical and biotechnology industry, where he has assisted more than 200 health care firms in a wide range of validation, automation, and compliance areas. Jim has over 45 years of industry experience and has worked in organic synthesis, pharmaceutical formulation, pharmaceutical production, project and process engineering, validation, and process automation during his career at Merck, Pfizer, Squibb and Bristol-Myers Squibb. He received his BS in chemical engineering from Pratt Institute, his MS in chemical engineering from Polytechnic Institute of New York, and his MBA in pharmaceutical studies from Fairleigh Dickinson University. He is a past president of the Parenteral Drug Association (PDA) and a member of USP’s Microbiology Expert Committee. He serves on the scientific advisory boards of MEDInstill and Eniware, and is a frequent author and lecturer on the subjects of sterilization, aseptic processing, and process validation.
James Agalloco is president of Agalloco & Associates, a technical service firm to the pharmaceutical and biotechnology industry, where he has assisted more than 200 health care firms in a wide range of validation, automation, and compliance areas. Jim has over 45 years of industry experience and has worked in organic synthesis, pharmaceutical formulation, pharmaceutical production, project and process engineering, validation, and process automation during his career at Merck, Pfizer, Squibb and Bristol-Myers Squibb. He received his BS in chemical engineering from Pratt Institute, his MS in chemical engineering from Polytechnic Institute of New York, and his MBA in pharmaceutical studies from Fairleigh Dickinson University. He is a past president of the Parenteral Drug Association (PDA) and a member of USP’s Microbiology Expert Committee. He serves on the scientific advisory boards of MEDInstill and Eniware, and is a frequent author and lecturer on the subjects of sterilization, aseptic processing, and process validation.
 David Hussong has 46 years of professional microbiology experience. Since 2015, he has been a senior consultant with ValSource, LLC, where he specializes in regulatory microbiology issues. David is retired from the Commissioned Corps of the U.S. Public Health Service after 30 years with the FDA. He is currently the chair of the USP Microbiology Expert Committee, a member of the PDA, and a member of the American Society for Microbiology. Dave earned his Ph.D. in microbiology from the University of Maryland (UM) and has also served as a research microbiologist at UM, the U.S. Department of Agriculture, and the U.S. Naval Medical Research Institute.
David Hussong has 46 years of professional microbiology experience. Since 2015, he has been a senior consultant with ValSource, LLC, where he specializes in regulatory microbiology issues. David is retired from the Commissioned Corps of the U.S. Public Health Service after 30 years with the FDA. He is currently the chair of the USP Microbiology Expert Committee, a member of the PDA, and a member of the American Society for Microbiology. Dave earned his Ph.D. in microbiology from the University of Maryland (UM) and has also served as a research microbiologist at UM, the U.S. Department of Agriculture, and the U.S. Naval Medical Research Institute.
 Leonard Mestrandrea is an established, broad-based pharmaceutical consultant with experience in industry, government, and academia. He possesses strong managerial and scientific experience in the areas of quality assurance, quality control, product quality performance, sterile manufacturing, and regulatory compliance, including NDA review and preparation. In addition to nine years of experience as the chief microbiologist within the FDA, he has more than 35 years of experience in the pharmaceutical industry. Leonard is experienced in the preparation for and conducting of PAIs, as well as GMP and foreign regulatory inspections, and he is well versed in issues of GMP and FDA compliance, microbiological analysis, and methods development. He received his BS from St. John’s University, an MS in microbiology from Wagner College, and a Ph.D. from Pacific Western University.
Leonard Mestrandrea is an established, broad-based pharmaceutical consultant with experience in industry, government, and academia. He possesses strong managerial and scientific experience in the areas of quality assurance, quality control, product quality performance, sterile manufacturing, and regulatory compliance, including NDA review and preparation. In addition to nine years of experience as the chief microbiologist within the FDA, he has more than 35 years of experience in the pharmaceutical industry. Leonard is experienced in the preparation for and conducting of PAIs, as well as GMP and foreign regulatory inspections, and he is well versed in issues of GMP and FDA compliance, microbiological analysis, and methods development. He received his BS from St. John’s University, an MS in microbiology from Wagner College, and a Ph.D. from Pacific Western University.
