Tetrapeptide Caspase Substrates: Their Uses and Limitations
By Konrad T. Howitz, Ph.D., BIOMOL Research Laboratories, Inc.
A well-established "symptom" of mammalian cell apoptosis is the ability of cell extracts to cleave chromogenic or fluorogenic substrates that include the sequence DEVD1. The DEVD sequence derives from the caspase-3 cleavage site in poly(ADP-ribose) polymerase (PARP)2 While caspase-3 is often the primary source of cellular "DEVDase" activity, other "effector" caspases, such as caspase-7 or -6, may also contribute3. Cell-extract DEVDase assays provide reliable, convenient and early detection of apoptosis (Figure 1).
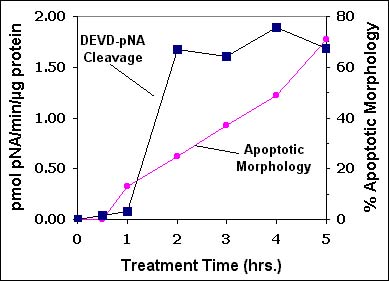
Figure 1. DEVD-pNA Cleaving Activity in Extracts of Apoptotic U937 Cells. Apoptosis was induced in U937 cells by treatment with 1 µg/ml soluble TRAIL (cat.# SE-721). At the times indicated, cells were lysed and DEVDase activity was measured using the BIOMOL QuantiZyme Assay System, Caspase-3 Cellular Activity Kit (cat.# AK-703. Apoptotic morphology was assessed as cells with two or more membrane "blebs".
As the list of known caspases has grown (14 at last count), efforts have been made to identify efficiently cleaved tetrapeptide substrates for the various caspase family members4,5. Some sequences, such as IETD for caspase-86 or VEID for caspase-67,8 derive from known in vivo protein targets. Others, such as LEHD for caspase-9, were generated in vitro, by combinatorial studies5. Such substrates are, of course, useful for assaying purified caspase enzymes. It should be noted, however, that there is substantial overlap in the tetrapeptide substrate preferences of the caspases (Figure 2). Since an apoptotic cell-extract may contain a mixture of several activated caspases, assays based on the cleavage a single tetrapeptide substrate, under just one set of conditions, cannot produce a valid measure of the activity of any particular caspase. In other words, for cell-extracts, DEVDase does not necessarily equal caspase-3, nor VEIDase caspase-6, nor IETDase caspase-8 and so forth.
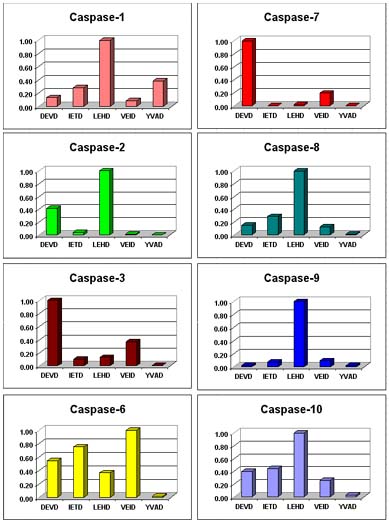
Figure 2. Cleavage of Tetrapeptide-pNA's by Recombinant Human Caspases. Relative rates of tetrapeptide-p-nitroaniline (pNA) cleavage were determined with 200 µM of the indicated substrate-pNA.
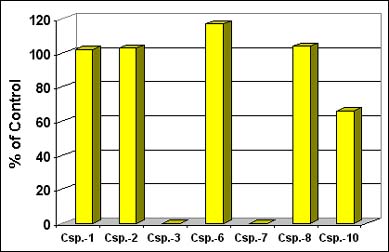
Figure 3. Effect of Casputin Reagent on the Activity of Recombinant Caspases. Initial rates of Ac-IETD-AMC cleavage (75 µM; cat.# P-432) were determined at 37°C, with 30 U of each enzyme, +/- 90 µg/ml Casputin (cat.# SE-760), with a microplate fluorimeter (Ex.: 360 nm; Em.: 460 nm).
The differences in substrate preference, kinetics and inhibitor sensitivities among the caspases are sufficient that, in principal, the data from multiple cleavage assays could together be used to identify all the caspases in a cell extract. Such an approach would need to include: 1) at least as many independent assay conditions as there are active species in the extract; 2) data from the pure enzymes obtained under the same conditions. Even a partial application of this approach can yield useful information. The Casputin Reagent is a potent and specific inhibitor of caspases-3 and -7 (Figure 3). Measuring IETD-AMC cleavage and DEVD-AMC, in both absence and presence of Casputin generates four independent assay conditions. Data gathered under these conditions, from extracts of TRAIL-treated Jurkat cells, reveals IETD-AMC cleaving activities that had been buried in a background of caspase-3 and -7 activities (Figure 4; compare A and B for times 45 min.-120 min.).
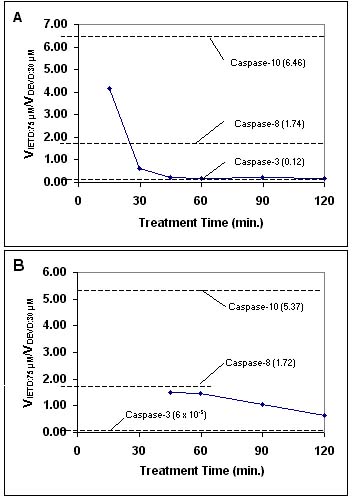
Figure 4. Ratio of the Rates of IETD-AMC to DEVD-AMC Cleavage by Extracts of TRAIL-Treated Jurkat Cells: The Effect of Casputin Reagent. Jurkat cells (diamonds/solid lines) were treated with 2 µg/ml soluble human TRAIL (cat.# SE-721). Assays were performed at 37°C, with 75 µM IETD-AMC or 30 µM DEVD-AMC and 0.2 mg/ml cell extract protein. Dashed lines and numbers in parentheses indicate rate ratios for human recombinant caspases, assayed under the same conditions as the cell extracts. A. Ratios with no added inhibitor. B. Ratios in the presence of 80 µg/ml Casputin (cat.# SE-760).
REFERENCES:
- N.A. Thornberry and Y. Lazebnik Science 1998 281 1312
- Y.A. Lazebnik et al. Nature 1994 371 346
- L. Faleiro et al. EMBO J. 1997 16 2271
- R.V. Talanian et al. J. Biol. Chem. 1997 272 9677
- N.A. Thornberry et al. J. Biol. Chem. 1997 272 17907
- D.W. Nicholson et al. Nature 1995 376 37
- A. Takahashi et al. Proc. Natl. Acad. Sci. USA 1996 93 8395
- W.D. Jarvis et al. J. Biol. Chem. 1996 271 8275
