Pharmaceutical Ultrapure Water Systems: Valuable Lessons Learned From Other Industries
By Igor Gorsky, ValSource, Inc.
 In August 2002, the U.S. Food and Drug Administration (FDA) embarked on an initiative entitled Pharmaceutical CGMPs for the 21st Century ― A Risk-Based Approach.1 This initiative particularly emphasized the FDA’s recommendations regarding use of technological advances in pharmaceutical manufacturing, as well as implementation of modern risk management and quality system tools and concepts. The FDA’s continuous striving towards the goals outlined in this initiative resulted in the issuance of a number of guidances that provide industry with better tools towards modernization. These include guidance documents on process analytical technology (PAT)2 and process validation3.
In August 2002, the U.S. Food and Drug Administration (FDA) embarked on an initiative entitled Pharmaceutical CGMPs for the 21st Century ― A Risk-Based Approach.1 This initiative particularly emphasized the FDA’s recommendations regarding use of technological advances in pharmaceutical manufacturing, as well as implementation of modern risk management and quality system tools and concepts. The FDA’s continuous striving towards the goals outlined in this initiative resulted in the issuance of a number of guidances that provide industry with better tools towards modernization. These include guidance documents on process analytical technology (PAT)2 and process validation3.
Although we have seen dramatic increase in use of new technologies and various PAT implementations, they still account for only a moderate segment of pharmaceutical facilities. More often, we hear from the podiums of pharmaceutical technology forums strong remarks about antiquated facilities, utilities, and, therefore, processes. In the last few years, we all witnessed an increase in regulatory reprimand — and even closure — of numerous manufacturing plants due to aging facilities and poor planning on behalf of their management for future advancements. This was actually one of the discussion subjects at the 2015 PDA Annual Meeting, where a representative from the FDA’s Office of Regulatory Affairs presented a review of inspection trends related to aging facilities.4
Furthermore, recent investigation by FDA into a multistate outbreak has identified the water-based bacteria Burkholderia cepacia in more than 10 lots of oral liquid docusate sodium linked directly to an aged water system at a contract manufacturing organization’s (CMO) Florida-based site. 5 This is an example of how serious the situation has become in an industry where manufacturers often seem to favor proven, older technologies over newer ones.
There are many reasons for this resistance to change, including the perceived hurdle of lengthy regulatory filings and the requirement to explain new technologies to regulators during inspections. It is sad to note that a considerable segment of manufacturers do not consider the implementation of new technologies as an important enabler or catalyst for the introduction, optimization, and upkeep of more robust manufacturing processes.
Pharmaceutical water technology is no exception in the long list of tools that have been used for at least 30 years. While other industries — many of which started their growth much later than pharmaceutical industry — continuously explore new ways of robust water treatment, we still seem reluctant to learn from them.
The semiconductor industry started its meteoric growth in mid-1960s. Very early in its infancy, one of its pioneers, Intel Corporation and Fairchild Semiconductor cofounder Gordon E. Moore, made an interesting observation, which he described in a landmark 1965 paper. This observation, now called Moore’s Law, basically says that the number of transistors that can fit on an integrated circuit doubles approximately every two years. This presents a continuous challenge for this industry — to effectively and efficiently use and optimize new and improved technologies. These technologies include improved water systems, as ultrapure water is used to rinse manufactured chips.
There is much the pharmaceutical industry can learn from the water technology advancements implemented in the semiconductor industry. We begin with an ultrapure water production technology retrospective for the last 20 to 30 years. Table 1 illustrates advances in ultrapure water production used in electronic chip manufacturing over that span of time.
Table 1: Comparison of Ultrapure Water Production, Past and Present
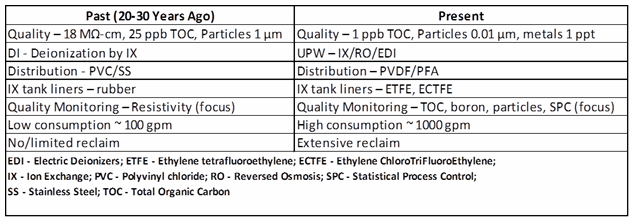
The left column of Table 1 essentially illustrates the usual current pharmaceutical water system. Few advances in ultrapure water technology have been introduced in pharmaceutical and biopharmaceutical industries over the past 20 to 30 years, while considerable advances have been made in the microelectronics industry.
Next, Table 2 examines, side-by-side, the water systems requirements currently utilized in each industry.5
Table 2: Ultrapure Water Requirements — Pharmaceutical vs. Semiconductor Industry
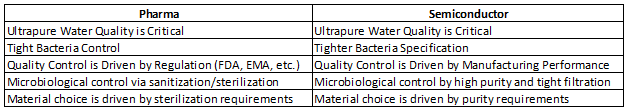
It is clear that we in the pharmaceutical industry typically tend to be reactive in our choice of purified water design, qualification, and subsequent use and management. The semiconductor industry, on the other hand, is driven by manufacturing performance, and therefore it is always on a lookout for improvements in quality.
Finally, we will examine a typical ultrapure water system diagram from a microchip manufacturing facility (Figure 1), to show some of the improvements that have been implemented in that industry.
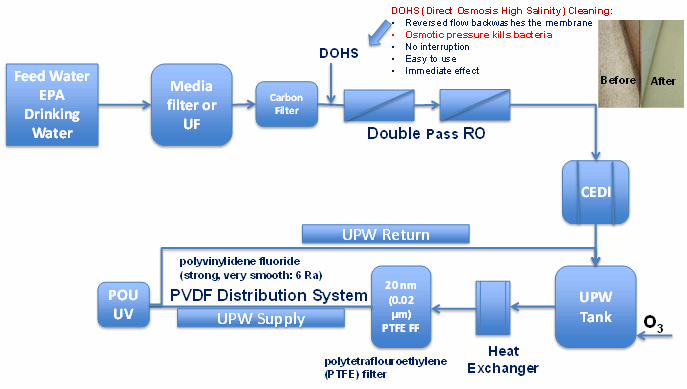
Figure 1: Typical semiconductor industry ultrapure water system schematics
Several important differences from typical pharmaceutical and biopharmaceutical water systems are noticeable in Figure 1:
- Use of double-pass reverse osmosis (RO) systems
- Utilization of direct osmosis high-salinity cleaning that allows effective in-place backwashes, which kill bacteria by osmotic pressure
- Use of new materials such as polyvinylidene fluoride, which is much smoother (6 microinch roughness average) than a stainless steel surface and just as strong
- Filtration through a 20 nm (0.02 µm) polytetrafluoroethylene (PTFE) filter
- Ozonation
Some of these innovations, such as ozonation, are used in our industry, though scarcely. However, we are less than open to further water purification innovations that would promote prevention rather than reaction measures.
Furthermore, the following recommendations from the semiconductor industry may present viable opportunities for future ultrapure water installations, as well as for renovated and updated systems, at pharmaceutical manufacturing facilities:
- Cost and effort can be reduced by changing operating/design philosophy from reactive to proactive, based on production of water with low total organic carbon (TOC) content.
- Double-pass RO is a proactive design option that produces low-TOC water, thus reducing bioburden proliferation.
- Inexpensive TOC control includes multiple inline TOC instruments in strategic locations that provide continuous live feedback for systems, thus increasing proactive control
- Limiting “food” for microorganisms is less expensive than “killing” them. Reducing TOC content of water — rather than acknowledging and continuously struggling with biofilm formations —has become the subject of guidances from both industry organizations6 and regulators7.
- Particle removal provides effective microbiological control, as shown on the low TOC content of ultrapure water used in semiconductor industry, which directly relates to low bioburden.
Pharmaceutical manufacturers can definitely learn a great deal from the improvements made in industries, such as microchip manufacturing, when it comes to the design, installation, qualification, use, and management of ultrapure water systems. Providing system design for a tighter quality control in turn will deliver more reliable, better quality performance.
References:
- FDA, Pharmaceutical cGMPS for the 21st Century — A Risk-Based Approach: Second Progress Report and Implementation Plan, updated on June 10, 2015.
- FDA, Guidance for Industry: PAT — A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance, September 2004.
- FDA, Guidance for Industry: Process Validation: General Principles and Practices, January 2011.
- Sharon K. Thoma, Aging Facilities — Inspection Trends, PDA Annual Meeting, Las Vegas, March 2015.
- FDA, Updates on Multistate Outbreak of Burkholderia cepacia Infections, updated on Oct. 12, 2016.
- Vyacheslav (Slava) Libman, Ultrapure Water — Quality and Technology to Support Advanced Industries’ Needs, Interphex 2014, New York, March 2014.
- Parenteral Drug Association (PDA), PDA Technical Report No. 69: Bioburden and Biofilm Management in Pharmaceutical Manufacturing Operation, Bethesda, MD, June 2015.
- European Medicines Agency (EMA), Questions and answers on production of water for injections by non-distillation methods – reverse osmosis and biofilms and control strategies (draft), June 2016.
About The Author:
 Igor Gorsky (igorsky@concordiavalsource.com) has over 30 years of experience leading validation, technology transfer, quality assurance, and manufacturing functions in a wide range of pharmaceutical, biotechnology, and medical device products for generic and brand-name firms. Currently, Igor is a senior consultant at ConcordiaValsource, LLC. He is a frequent speaker/writer on topics such as cleaning validation, critical utilities, process scale-up and validation, and knowledge management. He is active in the Parenteral Drug Association (PDA), where he leads the Water Interest Group. Igor is a coauthor of PDA Technical Report 29: Points to Consider for Cleaning Validation and PDA Technical Report 60: Process Validation: A Lifecycle Approach. He holds a bachelor’s degree in electrical-mechanical engineering technology from Rochester Institute of Technology.
Igor Gorsky (igorsky@concordiavalsource.com) has over 30 years of experience leading validation, technology transfer, quality assurance, and manufacturing functions in a wide range of pharmaceutical, biotechnology, and medical device products for generic and brand-name firms. Currently, Igor is a senior consultant at ConcordiaValsource, LLC. He is a frequent speaker/writer on topics such as cleaning validation, critical utilities, process scale-up and validation, and knowledge management. He is active in the Parenteral Drug Association (PDA), where he leads the Water Interest Group. Igor is a coauthor of PDA Technical Report 29: Points to Consider for Cleaning Validation and PDA Technical Report 60: Process Validation: A Lifecycle Approach. He holds a bachelor’s degree in electrical-mechanical engineering technology from Rochester Institute of Technology.
Image credit: A UPW Installation using PVDF Piping, Wikikart99, 2009, via CC0 1.0.
