Cystis fibrosis transmembrane conductance protein regulator found
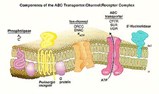
Accessory protein dimerizes CFTR in the membranes, improving its function and providing a new avenue for treating CF patients.
Scientists from Johns Hopkins University (Baltimore) report in the October issue of Cell on their finding of an accessory protein to the cystic fibrosis transmembrane conductance regulator (CFTR). Exploiting this newly found regulator of cystic fibrosis chemistry, they have been able to improve CFTR function, the molecule whose function is most affected by this common inherited disorder.
CFTR protein molecules form ion channels in the cells that line the lungs and other organ targets of cystic fibrosis, regulating the flow of ions in and out of the cells. Mutations in the gene are marked by accumulation of mucus and by abnormal sweat, digestive and other secretory glands.

The gene encoding a chloride ion channel is defective in patients with cystic fibrosis. (Q. Alawqati, Columbia University, NY, USA. Adapted by K. Sutliff, SCIENCE.)
"People with cystic fibrosis have abnormal chemistry outside of cells, not because their CFTR molecules won't work, but largely because they have too few of them on their cell membranes," says molecular biologist Min Li, who led the research team.
Studying the protein in human and mouse cell cultures and isolated cell membranes, Li and his co-workers showed that a regulatory cell protein they call CAP 70 lies adjacent to CFTR in cell membranes and binds to CFTR as well. Moreover, when CAP 70 is present, it links two CFTR molecules together, subtly changing the shape of each so they work together more efficiently.
"This counters the decades-old idea that CFTR exists only as single molecules," says Li. "There's a whole control system here we didn't know existed before."
Potentially, what this means is that in CF patients, existing channels could be made to work more efficiently.
"We might be able to compensate for their having too few of them," says Li. In one part of their research, for example, team members were able to double the flow of ions—charged atoms—through CFTR channels by adding CAP 70.
Beyond the potential for compensating for the disease's effect on target cells, the new work should quickly lead to improved screening of CF therapies, Li says. The traditional way to spot a useful drug, for example, has been a painstaking method isolating cell membranes and making measurements of minuscule changes in current.
"But now that we know that dimers—the double CFTR molecules—are the working version in cells, we can screen for them, and they're far easier to detect," Li says.
The research was funded by grants from the NIH, the Cystic Fibrosis Foundation and the American Heart Association. Other scientists on the paper were Shusheng Wang, Hongwen Yue, Rachel B. Derin, and William B. Guggino.
For more information: Min Li, Department of Neuroscience, The Johns Hopkins University School of Medicine, 725 North Wolfe St., Baltimore, MD 21205. Tel: 410-614-5131. Fax: 410-614-1001. Email: minli@jhmi.edu.
Edited by Laura DeFrancesco
Managing Editor, Bioresearch Online
ldefrancesco@bioresearchonline.com
