Apaf-1 switched off in malignant melanoma
Reactivating may provide a new approach to chemotherapy
Researchers from Cold Spring Harbor Laboratory (Cold Spring Harbor, NY) report in the January 11 issue of Nature that some malignant melanomas have lost the function of an apoptosis activation factor (Apaf-1), which explains why these tumors are so resistant to chemotherapy. While other aggressive tumors frequently have mutations in p53, which subverts the normal apoptotic pathway at an early step, cancer researchers have puzzled over the presence of normal p53 activity in malignant melanomas, a very aggressive tumor in its own right. Now, Scott Lowe and colleagues have not only discovered why, but also have revealed an interesting possibility for treatment.
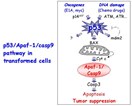
In an attempt to find the origins of malignant melanoma and to identify potential targets and strategies for therapy, Lowe examined the status of genes that function downstream of p53 in the pathway leading to apoptosis. When intact, this pathway rids the body of abnormal, pre-cancerous cells by triggering cell death. When this pathway is disrupted (by the loss of p53 function, for example), pre-cancerous cells survive and proliferate resulting in cancer (see figure).

Pathway leading to apoptosis or programmed cell death—DNA damage (such as that caused by certain chemotherapeutic drugs) and other signals stimulate p53. As a result, Apaf-1 is activated, triggering apoptosis. When this pathway is disrupted, as by loss of p53 or Apaf-1 function, pre-cancerous cells survive and proliferate, and eventually form a tumor. The pathway also functions naturally to generate shapes and control the number of cells in various tissues.
As is often the case in tumor cells, Lowe found that one of the two copies of the Apaf-1 gene was deleted in many of the melanomas they examined—a well-known phenomenon called loss of heterozygosity. However, the other Apaf-1 gene copy in these cells was present and apparently normal in terms of its DNA sequence. But in many of the melanoma lines, Apaf-1 expression was reduced compared to primary melanoma cells, leading them to suspect that the gene had been inactivated.
Since methylation is a common mechanism for inactivating genes, Lowe looked to see whether inhibiting methylation would affect expression levels of Apaf-1 in melanoma cells. After growing the cells for a few generations in 5 azacytidine, Apaf-1 expression levels were 10-20 fold higher.
"The loss of Apaf-1 in malignant melanoma is a prime explanation for both the extreme chemoresistance and the apparent lack of p53 defects in such cancers," says Lowe.
The study demonstrates that accurate diagnosis and treatment of malignant melanoma, and perhaps other cancers, should include an assessment of the status of Apaf-1. It also suggests that reversing the inactivation may be one way to approach treatment of this difficult cancer.
Maria Soengas of Cold Spring Harbor Laboratory was the principal author of the study. The principal collaborators in the study were William L. Gerald and Carlos Cordon-Cardo of Memorial Sloan-Kettering Cancer Center.
For more information: Scott Lowe, Professor, Cold Spring Harbor Laboratory, P.O. Box 100, 1 Bungtown Rd., Cold Spring Harbor, NY 11724. Email: lowe@cshl.org.
With contributions by Laura DeFrancesco
Managing Editor, Bioresearch Online
ldefrancesco@vertical.net
Source: Cold Spring Harbor Laboratories, Department of Public Affairs
