Analytical studies of four mammalian cell lines using the BioProfile 200
By Diane Wyatt, Gale Haslam, Jeff Knight, and Paul Kitos
Division of Biological Sciences, Kansas University (Lawrence)
Significant aspects of the metabolism of four cell lines in stationary culture were studied using the BioProfile 200 Chemistry Analyzer (N/A; Waltham, MA). Replicate cultures were prepared in 60 mm Tissue Culture dishes using D/F medium supplemented with either 5 or 10% FBS. D/F is a 1:1 mixture of DME and Ham's F12, and was reconstituted from powder (Sigma Chemical Co.; St. Louis). Two g/L of NaHCO3, plus 100 units of penicillin G and 100mg of streptomycin sulfate per mL of medium were added.
The inocula were prepared at either 5 x 104 or 1x 105 cells per dish, depending on the cell line. Cells from confluent cultures were grown in the same medium, suspended using trypsin: EDTA (0.25% trypsin 1:250 plus 0.1% sodium EDTA in PBS minus Ca++ and Mg++), counted using a Casy 1 particle sizer and counter, and seeded at the appropriate density in seven replicate 60 mm dishes. A second set of dishes (controls) was set up in identical fashion except that these dishes contained no cells. The dishes were incubated at 37°C in a humidified incubator flushed with 5% CO2 and 95% air. Each day, one dish without cells and one dish with cells was removed from the incubator, and the culture media were immediately collected in 5 mL Cryotubes, then sealed and frozen. At the same time, the cells were detached from the dish with trypsin:EDTA, and then counted and sized with the Casy 1 counter. After the cultures were taken, the accumulated media samples were thawed and analyzed with the BioProfile 200.
Results
In order to provide physiological profiles of several types of mammalian cells in culture, we examined four lines under standard culture conditions. The four cell lines and their serum supplements are listed below:
- HeLa monolayer (human cervical carcinoma), 5% FBS (Figures 1 a-d);
- CHO K1 (Chinese hamster ovary), 5% FBS ;
- NHEK (normal human epidermal keratinocytes), 5% FBS; and
- BBE (bovine brain endothelial), 10% FBS.
The first three are well known, widely used, anchorage-dependent, continuous cell lines capable of growing to high cell density. The fourth is a line of normal diploid bovine brain vascular endothelial cells that is subject to contact inhibition of growth.
For each cell line, the following information was obtained:
- the kinetics of cell proliferation, obtained by Casy 1 particle counting;
- the dynamics of change of glucose and lactate in the medium of both the control (no cells) and experimental (with cells) cultures; and
- similar information for glutamine, glutamate, ammonium, and calcium.
Other pieces of information available from the BioProfile analyses (Na+ and K+ concentrations, pH, pCO2, and pO2) are not included in this report.
See Figures 1a, 1b, 1c, and 1d for representative growth curves.
Cell proliferation
All four cultures exhibit typical exponential patterns of growth (see Figure 1a for a representative growth curve). In the seven-day period of the study, CHO K1 and NHEK cell increased from 5x 104 cells per dish to approximately 1x 107 cells per dish. HeLa cells grew somewhat more slowly, increasing from 5 x 104 to approximately 2.2 x 106 cells per dish in the same time span. It is notable that the volume of a HeLa cell is 3-4 times that of either the CHO or NHEK cell. Thus, the total increase in cell mass in these three cases is about the same. The BBE cultures were seeded at twice the density of the other three cultures (1 x 105 cells per dish), and in seven days increased to only 1.6 x 106 cells per dish, a reflection of the contact sensitivity of these cells.

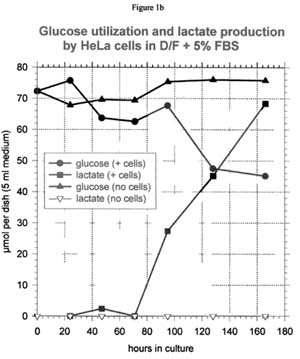
Glucose and lactate
In the absence of cells, there is no net change in the amount of glucose and lactate in the medium (see Figure 1b for a representative curve). In the presence of cells, lactate accumulates in the medium as glucose is consumed. Except for the NHEK cells, each mole of glucose used is accompanied by approximately 2 moles of lactate produced, which is the theoretical maximum. For NHEK cells, only about 1.2 moles of lactate accumulate per mole of glucose used.
Glutamine, glutamate, and ammonium ion
Glutamine is rapidly consumed by all four cell lines (see Figure 1c for a representative curve). In the case of HeLa and BBE cells, a small amount of glutamine may accumulate in the medium as glutamate. However, the glutamate content of the NHEK culture medium does not change appreciably as the glutamine is consumed. In the CHO culture medium, exogenous glutamate is actually depleted by the cells even while glutamine is still available. In the absence of cells, glutamine disappearance from the medium is significant and is not accompanied by an increase in the glutamate concentration.
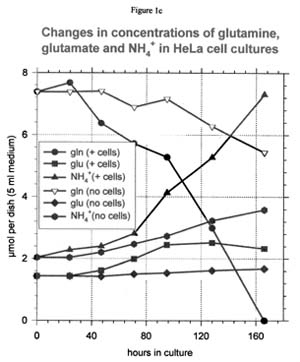
Thus, most of the non-cellular loss of glutamine must be due to its conversion to the nutritionally useless carboxypyrrolidone rather than to glutamate. The production of ammonium ion is essentially equivalent to the consumption of glutamine. This equivalence would be expected whether the used glutamine was converted to either glutamate or carboxypyrrolidone.
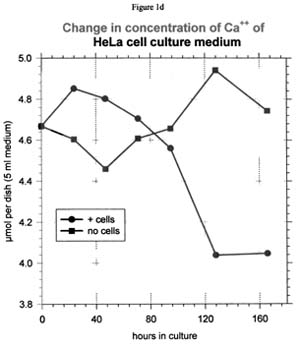
Calcium ion
It was surprising to find that that Ca++ was consumed in measurable amounts in three out of the four culture systems tested here (see Figure 1d for a representative curve). BBE cells were the exception, although even with these cells it may be possible that Ca++ uptake was not detected because of the smaller cell population.
Conclusion
The BioProfile 200 Chemistry Analyzer is an instrument with great potential for scientists in cell culture, especially in pharmaceutical and medical fields of research. It can provide critical data that define cell personalities. The analyzer is easy to operate and gives reproducible results.
To obtain additional figures from this paper, including curves for all four cell lines, please contact Nova Biomedical at 800-458-5813.
