A Risk-Based Approach To Environmental Monitoring & Control For Pharmaceutical Distribution
By Carla Reed, president, New Creed LLC
By Carla Reed, New Creed LLC, and Laurent Foetisch, Supply Chain Operations SA
Over the past few decades, many companies have spent time and capital investment in assessing and mitigating risk factors across the extended supply chain. However, even for those companies that have designed and implemented state-of-the-art operational supply and logistics networks, there is always risk that products may be impacted by variations in temperature, humidity, or other deviations from ideal conditions at any stage across the chain of custody.
Rules and Regulations
Regulatory authorities require that the manufacturer ensures product quality not only during storage and transportation but until it is used by the patient. The new EU regulations on good distribution practices (GDPs) are becoming the standard for the global movement of pharmaceutical materials and products. These regulations enforce the same level of care that is required for current good manufacturing practice (cGMP) compliance into the storage and distribution environment. cGDP, with appropriate guidelines and best practices, has been widely adopted by many health authorities around the world. (For a list of relevant international cGDP regulations, see the References and Resources section below.)
Environmental monitoring during storage and transportation is a requirement of cGDP and should include evaluating and protecting the shipment from the impact of humidity, light, oxygen, shocks, pressure, vibrations and X-rays — in addition to time, temperature monitoring, and control.
From the time the shipment departs the point of origin until it arrives at the final destination, it could be exposed to many situations that could compromise the quality and integrity of the materials/product contained therein. These could include transportation delays, extended periods where the shipment is exposed to the elements (e.g., tarmac holding time for air shipments), misrouting and/or trans-shipment, misplacement in a transit storage location, customs inspections, and/or detentions. And in even the most well-planned shipment routes there is the probability of unexpected weather variations, which can impact shipment time and exposing shipments to less-than-ideal conditions. Irrespective of the detailed risk review and shipment planning process, a shipment may experience one or more temperature excursions.
An excursion (negative or positive temperature deviation) can cause a change in the state of the product, which will then have to be quarantined until a CAPA (corrective and preventive action) has been initiated, a root cause analysis completed, a risk assessment performed, and the CAPA closed. In a best-case scenario, the shipment can be released but could have experienced delays of several weeks or even longer.
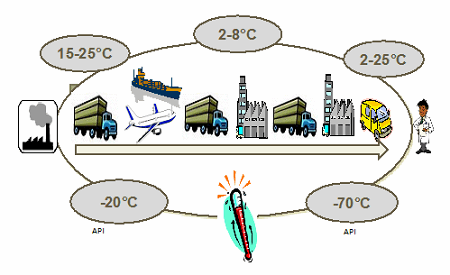
Figure 1: Biopharma products require multiple temperature-controlled ranges
Issues and Concerns
A major issue facing pharmaceutical companies, patients, and healthcare providers is a shortage of critical medicinal products. It is the duty of pharmaceutical manufacturers (including contract manufacturers) to decrease the risk of quality defects occurring while their products are in transit to manufacturing operations (upstream supply chain) and on their way to the patients (downstream supply chain).
At this point in the product lifecycle, when the product has reached a saleable stage, significant investment has already been made in R&D, clinical trials, regulatory approval, production, and sales and marketing. Failing to deliver a product at the end of the value chain may have severe effects on the patient health, on the company name, and on top-line growth. Therefore, the main concern of the marketing authorization holder, its related supply chain partners, and health authorities is to guarantee that drug products (pharmaceutical, biotech, vaccines, or any other related API or ingredient) are delivered to point-of-use and/or to patients without loss of therapeutic properties.
Whatever clinical or commercial supply chain environment you operate in, shipping or storage temperature deviations can have a major cost impact. This can include stock outs, interruptions in production activities, and even effects on the patient — lack of product delivery or delays in material/product receipt can result in having to reschedule a patient treatment.
It is possible to reduce the impact of these supply chain interruptions, or avoid them altogether, by engaging with the product development and quality team early in the process. The more precise the product temperature specifications, the more difficult it will be to find an optimal logistics solution to guarantee product integrity — by ensuring that there is no deviation from the correct temperature range. There is a direct link between the product temperature label range, and the cost of performing logistics, which can represent between 2 to 5 percent of product costs. It is therefore recommended to include cycling studies as part of the product development process. The results of these studies will provide the necessary information to determine stability data, which is critical to retaining product quality and integrity throughout the product lifecycle.
Take Product and Transportation Modes into Account
Different products will require different temperature ranges, based on their stability profiles. Temperature ranges can vary from controlled room temperature (CRT) all the way down to deep frozen. It is also important to remember that too cold is as bad as too hot — therefore, specific temperature ranges should be established and planned for. Time and temperature sensitivity planning should take into account seasonal variations as well as the different climate zones a shipment may be exposed to during movement from origin to destination. It is also important to consider the transportation mode — and the impact of vibration, altitude, or other environmental hazards.
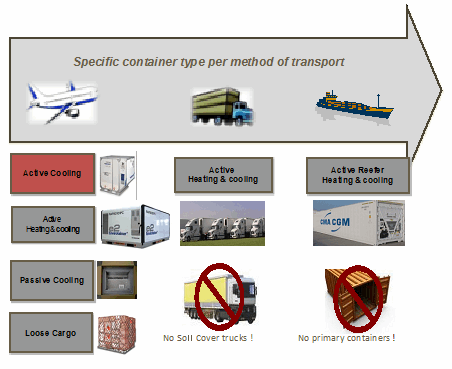
Figure 2: Transportation methods for primary or secondary distribution
Understanding the nature of the product — including stability data and any hazards that could have a negative impact on quality, safety, and efficacy — is critical for planning its storage and distribution. Factors to review and clarify in the early stages of the product lifecycle include: the protection that should be built into the packaging component, temperature management through the introduction of refrigerants, and any time, temperature, or other data logging requirements. Temperature requirements should be included in the product master data and clearly stated on product packaging, labels, and all documentation.
Evaluate Risk Factors and Design Product-Specific Mitigation Strategies
The European Commission’s revised GDP guideline (2013/C 343/01) provides the following recommendation for the distribution of time and temperature sensitive materials and products: “Risk assessment of delivery routes should be used to determine where temperature controls are required.”
 A team of cross-functional subject matter experts should map out the shipment process, identifying areas of potential risk, delay, deviations, or disruptions from the proposed transportation plan.
A team of cross-functional subject matter experts should map out the shipment process, identifying areas of potential risk, delay, deviations, or disruptions from the proposed transportation plan.
One of the first steps of the risk assessment is to perform shipping studies, mimicking temperature deviations — upwards and downwards — prior to engaging in the normal stability studies protocol. It is then advisable to run your normal stability protocol (stress, accelerated, or real-time) and confirm that the temperature exposures have no effect on the quality of drug product. These stability studies must be performed in accordance with the appropriate regulations and guidelines (ICH, WHO, USP, PDA, MHRA, FDA, and others).
During the risk assessment, it is appropriate to take into account the following elements that could have a negative — or positive — impact on the outcome of the transportation.
People
During shipment planning, it is necessary to consider the different organizations and their representatives that will be responsible for the safe and secure movement of the shipment. When conducting shipping studies, it is important to consider the impact of time on the tarmac in the case of ground-handling delays. Providing adequate thermal protection — including the introduction of phase change materials, refrigerants, and temperature-controlled shipping containers — will ensure that delays and exposure to variations in temperature will not impact the products being shipped.
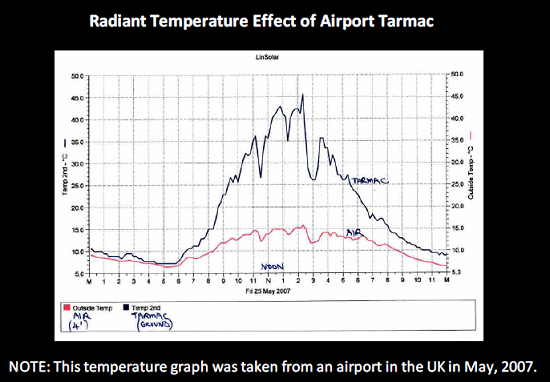
Figure 3: Ground-handling delays can increase time on tarmac, which can have a major impact on temperature-sensitive materials.
It is equally important to ensure that the correct level of documentation and labeling is provided, taking into account different languages and levels of literacy across the shipment lifecycle. Many logistics solutions providers are active in this field, as the market for temperature sensitive solutions continues to grow faster then any other industry segment. Reviewing requirements and options as part of the shipment planning process should also include discussions with logistics service providers, which can provide a variety of integrated solutions.
Process
Mapping out each step in the process across the various links in the chain of custody — with planned and potential elapsed time between each step — will assist in identifying hazards, risks, and mitigation strategies that should be integrated into the shipment plan. When defining the process steps, take into account trans-shipment points and changes in the chain of custody. Understanding each of the handoff points is critical; the weakest link in the chain is, in many cases, related to time between transit (e.g., time on the runway for air shipments or time at receiving docks during intermodal movements).
Technology
The combination of time in transit, stability data, and potential variations in temperature could require special packaging. Packaging technology is evolving quickly, and smarter solutions are being developed to address the challenges of temperature control. There are many different packaging options — active, advanced-passive, and passive — that should be evaluated based on the stability data gathered during product development. Options include everything from expensive “heat and cool” active containers to passive containers using phase-change cooling materials.
Maintaining a frozen temperature range is probably the easiest one to accomplish, since freezing/deep-freezing and maintaining a product at -20°C or lower can be achieved more easily than ensuring controlled room temperature (CRT) is maintained across a variety of temperature zones. It is more important to define and communicate acceptable ranges for temperature variations or excursions.
Monitoring these variations — and monitoring frequency and elapsed time for excursions — can be achieved through the use of data loggers. These can be included in the shipment (number and location to be determined based on size, density, and other factors). Ensuring that the shipment retains its integrity through a combination of packaging, refrigerants, and/or active refrigerated shipping containers is critical. Time and temperature data loggers actively track the location and state of the product, enabling intervention in the event of an excursion. In situations where it is impossible to intervene during transit, the data captured during the shipment lifecycle can provide the information needed to establish if there was an excursion from the desired temperature range. Again, the most important factor is to ensure the safety, integrity, and efficacy of the product.
This risk based approach to shipment planning also addresses regulator concerns related to the diversion or adulteration of the materials being transported. Defining each of the steps across the chain of custody and ensuring that a mechanism is in place to monitor and mitigate will provide a framework to ensure the state and location of the materials and/or product are known — and that any negative impact from temperature or other deviations can be remediated.
Risk factors can be evaluated and mitigation strategies integrated into the shipping execution plan, providing the appropriate packaging, labeling, and documentation required to ensure that materials and products move smoothly from origin to final destination.
Conclusion
Whatever the means of transportation — road, ocean, air, or a multimodal combination — it is critical to ensure that the shipment is transported across the upstream and downstream chain of custody in compliance with the correct temperature and transportation conditions. The appropriate shipment handling conditions should be reflected on all package labels, shipping documentation, and other materials that define the transportation and material handling conditions. Planning for any eventuality, and protecting the contents of the shipment while in transit, is key to ensuring that the products delivered to patients are of the highest quality, safe, and effectiveness.
References and Resources:
- ICH Q1A(R2): Stability Testing of New Drug Substances and Products, 2003
- Guidelines of 7 March 2013 on Good Distribution Practice of Medicinal Products for Human Use (2013/C 68/01), Official Journal of the European Union
- <1079> Good Storage and Distribution Practices for Drug Products, USP
- Model guidance for the storage and transport of time- and temperature-sensitive pharmaceutical products – Annex 9, WHO Technical Report Series, No. 961, 2011
- Guidance for Temperature-Controlled Medicinal Products: Maintaining the Quality of Temperature-Sensitive Medicinal Products Through the Transportation Environment PDA Technical Report 39, 2007
- Last Mile: Guidance for Good Distribution Practices for Pharmaceutical Products to the End User, PDA Technical Report 46, 2010
- Guidance for Good Distribution Practices (GDPs) For the Pharmaceutical Supply Chain, PDA Technical Report 52, 2011
- Guidance for Industry: Stability Testing to Support Distribution of New Drug Products, PDA Technical Report 53, 2011
- Risk Management for Temperature- Controlled Distribution, PDA Technical Report 58, 2012
- Stability testing of active pharmaceutical ingredients and finished pharmaceutical products – Annex 2, WHO Technical Report Series, No. 953, 2009
- A mathematical approach to assessing temperature excursions in temperature controlled chains, Claude Ammann, European Journal of Parenteral & Pharmaceutical Sciences, 13(2): 57-59, 2008
- Stability Studies Needed to Define the Handling and Transport Conditions of Sensitive Pharmaceutical or Biotechnological Products, Claude Ammann, AAPS PharmSciTech, Vol 12 number 4, Dec. 2011
- Handling Temperature Excursions and the Role of Stability Data, Claude Ammann, Pharmaceutical Outsourcing, Sep. 2013
About The Authors:
 Carla Reed is a seasoned supply chain professional with more than 25 years of experience providing leadership and program management across a variety of programs for the life sciences industry. Her broad range of experience and expertise has provided solutions for pharmaceutical and biotech companies challenged by the growing complexity of extended supply chain environments. Her firm, New Creed LLC, provides change leadership to facilitate sustainable solutions, providing hands-on experience in all aspects of supply chain operations. You can email her at newcreed11@gmail.com or connect with her on LinkedIn.
Carla Reed is a seasoned supply chain professional with more than 25 years of experience providing leadership and program management across a variety of programs for the life sciences industry. Her broad range of experience and expertise has provided solutions for pharmaceutical and biotech companies challenged by the growing complexity of extended supply chain environments. Her firm, New Creed LLC, provides change leadership to facilitate sustainable solutions, providing hands-on experience in all aspects of supply chain operations. You can email her at newcreed11@gmail.com or connect with her on LinkedIn.
 After spending more than 25 years as global VP of supply chain within a large European biopharmaceutical company, Laurent Foetisch founded Supply Chain Operations SA in 2011. The company, which serves as an experienced Swiss operational partner for supply chain operations, is initially focused on biotech and pharmaceutical value chain management but can benefit any process-based organization. Laurent studied economics and finance at Ecole de Commerce de Lausanne (Switzerland).
After spending more than 25 years as global VP of supply chain within a large European biopharmaceutical company, Laurent Foetisch founded Supply Chain Operations SA in 2011. The company, which serves as an experienced Swiss operational partner for supply chain operations, is initially focused on biotech and pharmaceutical value chain management but can benefit any process-based organization. Laurent studied economics and finance at Ecole de Commerce de Lausanne (Switzerland).
