A Novel, Fluorescence Polarization- Based Assay for Detecting Tyrosine Kinase Activity
Contributed by Gregory Parker and Randy Bolger, PanVera Corp. (Madison, WI)
Contents
Introduction
What Is Fluorescence Polarization?
Assay Theory
Results and Discussion
Introduction (Back to Top)
The phosphorylation of proteins by protein tyrosine kinases (PTK) is critical to the normal regulation of many biological mechanisms, including cell growth, proliferation, differentiation, and metabo-lism. Defects in these pathways can result in a number of human diseases, including cancer. Conventional tyrosine kinase assays are tedious, radioactive, and are not easily automated or converted to a high-throughput format for drug screening. The Protein Tyrosine Kinase Assay Kit described here is a major advance because it is simple, sensitive, inexpensive, nonradioactive, nonimmobilized, and formatted for high-throughput screening.
What Is Fluorescence Polarization? (Back to Top)
Fluorescence polarization (FP) is a technique for monitoring molecular interactions in a homogenous environment at equilibrium. The binding of a small, fluorescent molecule to another, larger molecule can be quantified by a change in the rate of rotation of the fluorescent molecule. Plane-polarized light is used to excite fluorescent molecules in solution. If the excited molecule remains stationary during the period of excitation (4 nanoseconds for fluorescein), the emitted light will remain highly polarized. However, if the excited molecule can tumble or rotate during this period, the emitted light will be depolarized.
Since FP is a measure of this tumbling rate, it is directly related to the molecular volume of the fluorescent molecule. An increase in molecular volume (due to biological processes such as receptor-ligand binding, antibody-antigen binding, DNA hybridization, or DNA-protein binding), or a decrease in molecular volume (due to enzymatic degradation or molecular dissocia-tion) can be measured directly by FP. The measured FP value is the weighted average of the FP values of the individual bound and free fluorescent molecules and is therefore a direct measure of the fraction bound. These data can be handled exactly the same way as traditional radioligand-binding assay data. Polarization is plotted against the logarithm of the receptor concentration, resulting in the common saturation-binding isotherm.
Assay Theory (Back to Top)
FP can be exploited in a PTK assay because fluorescein-labeled phospho-peptides (F-phosphopeptides) and phosphopeptides or phosphoproteins, which can be generated during a kinase reaction, will compete for binding to anti-phosphotyrosine antibodies (anti-pY Ab). When no kinase reaction products are present, a significant portion of the F-phosphopeptide tracer will be bound by the anti-pY Ab, resulting in a high polarization. However, after a PTK reaction has occurred, the anti-pY Ab will bind to both the reaction products and the F-phospho-peptide tracer, increasing the amount of free tracer and decreasing the fluorescence polarization of the sample. If enough competitor phosphopeptide is generated during the PTK reaction, the fluorescent tracer can be completely displaced from the anti-pY Ab and the emitted light will be totally depolarized. Thus, the change in FP is directly related to the amount of PTK activity. This is diagrammed in Figure 1. Depending on the instrumentation and reagents used, these measurements can be made in real time, allowing the observed enzymatic activity to be monitored kinetically.
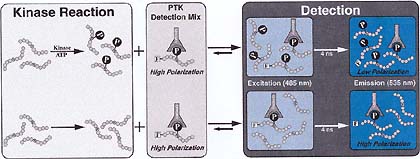
Figure 1
Results and Discussion (Back to Top)
The practical steps required to perform the FP-based PTK assay are outlined in Figure 2.
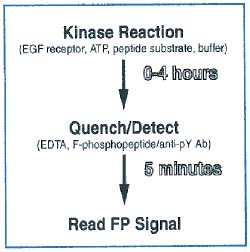
Figure 2
As shown in Figure 3, when all of the reaction and detection components are present, the polarization shifts from 160 mP to 91 mP over 1 hour. Initially, all of the F-phosphopeptide tracer is bound by anti-pY Ab, and therefore has a high polarization. However, as the PTK reaction proceeds and more competitor phospho-tyrosines are produced, the F-phospho-peptide is displaced from the anti-pY Ab and the polarization is reduced. Over the next 3 hours, the polarization drops from 91 mP to 77 mP as the PTK reaction continues. The maximum and minimum polarization values possible in this specific assay are indicated by two control reactions.
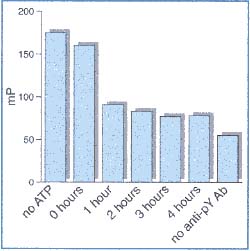
Figure 3
When there is no ATP present, the PTK reaction cannot proceed, there is no competition, and the F-phosphopeptide remains bound to the anti-pY Ab. The polarization remains high (175 mP) and stable. If the anti-pY Ab is not added to the quenched reaction, then the F-phospho-peptide/ anti-pY Ab complex cannot form, and the polarization remains low (55 mP).
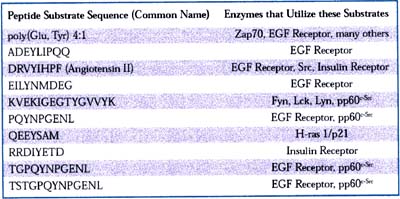
Table 1
These data demonstrate that an assay based on fluorescence polarization can be used to detect tyrosine kinase activity. This assay is simple, rapid and inexpensive. Its versatility is shown in Table 1. By adding unknown compounds to the kinase reaction, inhibitors or activators can be easily screened and IC 50 values can be established, as shown in Figure 4.
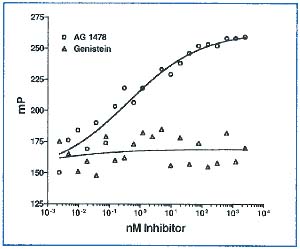
Figure 4
The screening of unknown kinases, the optimization of substrates, and the testing of reaction conditions are all possible with this assay. The general principles described here may also be applied to a tyrosine phos-phatase assay, which could be a powerful tool in a quality control laboratory setting. And finally, the FP application described here is being specifically extended by PanVera to serine/threonine kinases. It may also be broadly applied to any other modification of a peptide, small protein, or other molecule that can be fluorescently labeled and recognized by an antibody.
For more information: PanVera Corp., 545 Science Dr., Madison, WI 53711. Tel: 608-233-9450. Fax: 608-233-3007.
